|
| Postoperative
Complications |
Respiratory Care|
Cardiovascular Therapy|
Fluid Management| Nutrition in Critical Patients
|Traumatic Brain Injury-Stroke-Brain Death
|

I. Complications
after operation for supratentorial tumor
 Increased intracranial
pressure (ICP). The cranial cavity has a
limited capacity to accommodate increased
intracranial volume without a significant increase
in pressure. Increased volume of any of the three
components of the intracranial cavity ”brain cells,
blood, and cerebrospinal fluid (CSF)” may increase
ICP. Increased ICP causes injury to the brain by
compression, herniation, and ischemia. Brain
ischemia in turn enhances brain edema, propagating a
cycle of vascular insufficiency and swelling.
Increased intracranial
pressure (ICP). The cranial cavity has a
limited capacity to accommodate increased
intracranial volume without a significant increase
in pressure. Increased volume of any of the three
components of the intracranial cavity ”brain cells,
blood, and cerebrospinal fluid (CSF)” may increase
ICP. Increased ICP causes injury to the brain by
compression, herniation, and ischemia. Brain
ischemia in turn enhances brain edema, propagating a
cycle of vascular insufficiency and swelling.
Intracranial hemorrhage, hydrocephalus, and cerebral
edema are the most common postoperative causes of
increased ICP. Intracranial hypertension leads to
headache, nausea, vomiting, decreased level of
consciousness, and neurologic dysfunction. These
signs are not specific for increased ICP and are not
uncommon postoperatively. Detection of increased ICP
relies on clinical signs and symptoms, direct
measurement of ICP, and imaging studies such as
computed tomographic (CT) scanning. Prevention and
treatment seek to avoid and alleviate factors that
can aggravate intracranial hypertension such as
arterial hypertension, impaired cerebral venous
drainage, blocked or malfunctioning surgical drains,
postoperative pain, respiratory depression, nausea
and vomiting, shivering, and seizures.
 Cerebral edema is
minimized by intraoperative and postoperative use of
dexamethasone, loading dose 10 to 20 mg
intravenously (i.v.); then 4 mg i.v. every 6 hours
and mannitol, loading dose 0.25 to 2 g/kg i.v. over
30 minutes; then every 6 hours as needed, with
careful monitoring of fluid intake and output and
control of blood pressure and central venous
pressure.
Cerebral edema is
minimized by intraoperative and postoperative use of
dexamethasone, loading dose 10 to 20 mg
intravenously (i.v.); then 4 mg i.v. every 6 hours
and mannitol, loading dose 0.25 to 2 g/kg i.v. over
30 minutes; then every 6 hours as needed, with
careful monitoring of fluid intake and output and
control of blood pressure and central venous
pressure.
 Cerebral venous drainage
is facilitated by head elevation to 30°, blood
pressure permitting.
Cerebral venous drainage
is facilitated by head elevation to 30°, blood
pressure permitting.
 Postoperative ventilation and
oxygenation should be monitored and
supplemental oxygen and ventilatory support should
be provided as needed.
Postoperative ventilation and
oxygenation should be monitored and
supplemental oxygen and ventilatory support should
be provided as needed.
 Pain is treated with small doses of fentanyl, 0.5 to
1 mcg/kg i.v. every 1 to 2 hours; morphine, 0.025 to
0.05 mg/kg i.v. every 1 to 2 hours; or
hydromorphone, 0.2 to 1 mg i.v. every 2 to 3 hours.
Pain is treated with small doses of fentanyl, 0.5 to
1 mcg/kg i.v. every 1 to 2 hours; morphine, 0.025 to
0.05 mg/kg i.v. every 1 to 2 hours; or
hydromorphone, 0.2 to 1 mg i.v. every 2 to 3 hours.
 Postoperative nausea and
vomiting are minimized by intraoperative use
of dexamethasone and 5HT3 receptor antagonists such
as dolasetron, 12.5 mg i.v. in adults and 0.35 mg/kg
i.v. in children; or ondansetron, 4 mg i.v. over 4
minutes for adults and children weighing >40 kg and
0.1 mg/kg i.v. over 4 minutes for children weighing
<40 kg.
Postoperative nausea and
vomiting are minimized by intraoperative use
of dexamethasone and 5HT3 receptor antagonists such
as dolasetron, 12.5 mg i.v. in adults and 0.35 mg/kg
i.v. in children; or ondansetron, 4 mg i.v. over 4
minutes for adults and children weighing >40 kg and
0.1 mg/kg i.v. over 4 minutes for children weighing
<40 kg.
 Postoperative shivering
is treated by gradually rewarming the patient and
administering small doses of meperidine, 12.5 mg
i.v.
Postoperative shivering
is treated by gradually rewarming the patient and
administering small doses of meperidine, 12.5 mg
i.v.
 Postoperative seizures
are treated with phenytoin, loading dose 10 to 20
mg/kg i.v. no faster than 50 mg/minutes (1,000 mg
over 20 minutes in typical adults) and then 5 to 7
mg/kg/day; or fosphenytoin, loading dose phenytoin
equivalent (PE) 15 to 20 mg/kg i.v. no faster than
100 to 150 mg/minute (1,000 mg PE over 10 minutes in
typical adults) and then 4 to 6 PE mg/kg/day.
Postoperative seizures
are treated with phenytoin, loading dose 10 to 20
mg/kg i.v. no faster than 50 mg/minutes (1,000 mg
over 20 minutes in typical adults) and then 5 to 7
mg/kg/day; or fosphenytoin, loading dose phenytoin
equivalent (PE) 15 to 20 mg/kg i.v. no faster than
100 to 150 mg/minute (1,000 mg PE over 10 minutes in
typical adults) and then 4 to 6 PE mg/kg/day.
II. Complications
after operation for infratentorial tumor
 Increased ICP.
The elastance of the infratentorial compartment is
greater than that of the supratentorial compartment,
which means that it takes smaller increases in
volume to produce significant increases in pressure.
Furthermore, increased pressure in the posterior
fossa is more life threatening because it can
compress or herniate the brain stem, which contains
the respiratory and vascular centers. Brain stem
herniation can occur downward through the foramen
magnum or upward through the tentorium, which is
most common after resection of tumors of the
cervical medullary junction. Brain stem compression
is manifested by a decreased level of consciousness
and respiratory and cardiovascular abnormalities
including collapse. Measures to control ICP should
be continued in the postoperative period: head
elevation; prevention and treatment of hypertension;
treatment of pain, nausea, and vomiting; prevention
and treatment of shivering; and maintenance of
adequate ventilation and oxygenation. Decreased
level of consciousness or the development of
respiratory or cardiovascular abnormalities should
prompt surgical consultation.
Increased ICP.
The elastance of the infratentorial compartment is
greater than that of the supratentorial compartment,
which means that it takes smaller increases in
volume to produce significant increases in pressure.
Furthermore, increased pressure in the posterior
fossa is more life threatening because it can
compress or herniate the brain stem, which contains
the respiratory and vascular centers. Brain stem
herniation can occur downward through the foramen
magnum or upward through the tentorium, which is
most common after resection of tumors of the
cervical medullary junction. Brain stem compression
is manifested by a decreased level of consciousness
and respiratory and cardiovascular abnormalities
including collapse. Measures to control ICP should
be continued in the postoperative period: head
elevation; prevention and treatment of hypertension;
treatment of pain, nausea, and vomiting; prevention
and treatment of shivering; and maintenance of
adequate ventilation and oxygenation. Decreased
level of consciousness or the development of
respiratory or cardiovascular abnormalities should
prompt surgical consultation.
 Brain stem injury
can occur intraoperatively. This presents
postoperatively as failure to regain consciousness
or resume spontaneous respiration with
cardiovascular abnormalities such as bradycardia and
hypertension/hypotension. Pharmacologic causes of
such manifestations should be ruled out by ensuring
adequate reversal of anesthetic agents and muscle
relaxants. Supportive care in the form of mechanical
ventilation and hemodynamic therapy is provided as
needed.
Brain stem injury
can occur intraoperatively. This presents
postoperatively as failure to regain consciousness
or resume spontaneous respiration with
cardiovascular abnormalities such as bradycardia and
hypertension/hypotension. Pharmacologic causes of
such manifestations should be ruled out by ensuring
adequate reversal of anesthetic agents and muscle
relaxants. Supportive care in the form of mechanical
ventilation and hemodynamic therapy is provided as
needed.
 Injury to cranial nerves
IX, X, and XII may compromise the
patient's ability to maintain a patent and protected
airway due to difficulty in swallowing and clearing
secretions. Tracheal extubation should be performed
only after the integrity of protective upper airway
reflexes is evident. After extubation, respiratory
monitoring should continue with readiness to
reintubate the trachea and reinstitute mechanical
ventilation if the patient fails to maintain
adequate ventilation and a patent and protected
airway. Injury to the ophthalmic division of the
trigeminal nerve (cranial nerve V) may impair
protective reflexes of the cornea and require
external protection with an eye patch.
Injury to cranial nerves
IX, X, and XII may compromise the
patient's ability to maintain a patent and protected
airway due to difficulty in swallowing and clearing
secretions. Tracheal extubation should be performed
only after the integrity of protective upper airway
reflexes is evident. After extubation, respiratory
monitoring should continue with readiness to
reintubate the trachea and reinstitute mechanical
ventilation if the patient fails to maintain
adequate ventilation and a patent and protected
airway. Injury to the ophthalmic division of the
trigeminal nerve (cranial nerve V) may impair
protective reflexes of the cornea and require
external protection with an eye patch.
 Edema of the mucosa
of the upper airway may occur after prolonged
surgery, especially in the sitting position. It is
more significant in children due to the small
diameter of their airways. Tracheal extubation
should be performed only after absence of airway
edema is ascertained by deflating the cuff of the
endotracheal tube and confirming the ability of the
patient to breathe around the tube. If airway edema
is suspected, the trachea should remain intubated
and the patient sedated, if needed, until the edema
resolves. Inhaled racemic epinephrine, 0.5 mL of 2%
solution in 3 mL saline, decreases localized mucosal
edema and might relieve upper airway obstruction.
Macroglossia may accompany upper airway edema,
causing complete airway obstruction. If this occurs,
cricothyroidotomy, tracheotomy, or insertion of
laryngeal mask airway may be the fastest way to
reestablish the airway.
Edema of the mucosa
of the upper airway may occur after prolonged
surgery, especially in the sitting position. It is
more significant in children due to the small
diameter of their airways. Tracheal extubation
should be performed only after absence of airway
edema is ascertained by deflating the cuff of the
endotracheal tube and confirming the ability of the
patient to breathe around the tube. If airway edema
is suspected, the trachea should remain intubated
and the patient sedated, if needed, until the edema
resolves. Inhaled racemic epinephrine, 0.5 mL of 2%
solution in 3 mL saline, decreases localized mucosal
edema and might relieve upper airway obstruction.
Macroglossia may accompany upper airway edema,
causing complete airway obstruction. If this occurs,
cricothyroidotomy, tracheotomy, or insertion of
laryngeal mask airway may be the fastest way to
reestablish the airway.
 Pneumocephalus
occurs after craniectomy, especially in the sitting
position, and is usually of little clinical
consequence. Tension pneumocephalus, which may
decrease the level of consciousness due to brain
compression, is more common in patients after
ventricular shunting and aggressive drainage of CSF,
which allows air trapping in the space that
surrounds the brain that has been drained of CSF.
Pneumocephalus is diagnosed by a CT scan or x-ray of
the head and is effectively treated with a burr
hole, which can be done under local anesthesia and
usually produces rapid recovery of consciousness
once the trapped air has been released.
Pneumocephalus
occurs after craniectomy, especially in the sitting
position, and is usually of little clinical
consequence. Tension pneumocephalus, which may
decrease the level of consciousness due to brain
compression, is more common in patients after
ventricular shunting and aggressive drainage of CSF,
which allows air trapping in the space that
surrounds the brain that has been drained of CSF.
Pneumocephalus is diagnosed by a CT scan or x-ray of
the head and is effectively treated with a burr
hole, which can be done under local anesthesia and
usually produces rapid recovery of consciousness
once the trapped air has been released.
 Patients who develop intraoperative venous air
embolism may subsequently develop postoperative
pulmonary edema
requiring mechanical ventilation and diuresis.
Extubation is performed after resolution of
pulmonary edema as documented by clinical
examination, chest x-ray, and arterial blood gases.
Patients with functionally patent foramen ovale may
develop paradoxical air embolism, which is manifest
postoperatively by neurologic deficits, decreased
level of consciousness, and cardiac abnormalities.
Patients who develop intraoperative venous air
embolism may subsequently develop postoperative
pulmonary edema
requiring mechanical ventilation and diuresis.
Extubation is performed after resolution of
pulmonary edema as documented by clinical
examination, chest x-ray, and arterial blood gases.
Patients with functionally patent foramen ovale may
develop paradoxical air embolism, which is manifest
postoperatively by neurologic deficits, decreased
level of consciousness, and cardiac abnormalities.
III. Complications
after operation for pituitary tumor
 Endocrine complications
include adrenocortical insufficiency,
hypothyroidism, and diabetes insipidus (DI).
Endocrine complications
include adrenocortical insufficiency,
hypothyroidism, and diabetes insipidus (DI).
 All patients receive corticosteroid coverage until
testing indicates an intact pituitary-adrenal axis.
All patients receive corticosteroid coverage until
testing indicates an intact pituitary-adrenal axis.
 Thyroid hormone replacement is reserved for patients
who were hypothyroid preoperatively.
Thyroid hormone replacement is reserved for patients
who were hypothyroid preoperatively.
 DI occurs in 10% to 20% of patients, usually
develops within 12 to 24 hours of surgery, and lasts
for a few days. Decreased release of antidiuretic
hormone results in the excretion of excessive
amounts (4 to 14 L/day) of dilute urine and leads to
dehydration, hypernatremia, increased serum
osmolality (>300 mOsm/kg), decreased urine
osmolality (<200 mOsm/kg), and decreased urine
specific gravity (<1.005). Symptoms of hypernatremia
are nonspecific and include decreased level of
consciousness, tremulousness, muscle weakness,
irritability, ataxia, spasticity, confusion,
seizures, coma, and possibly intracranial bleeding
due to increased serum osmolality. Treatment of DI
consists of hydration and hormonal supplementation.
The amount and content of intravenous fluids are
guided by urine volume, serum electrolytes, and
serum osmolality. Free water (H2O)
deficit can be estimated using the following
formula:
DI occurs in 10% to 20% of patients, usually
develops within 12 to 24 hours of surgery, and lasts
for a few days. Decreased release of antidiuretic
hormone results in the excretion of excessive
amounts (4 to 14 L/day) of dilute urine and leads to
dehydration, hypernatremia, increased serum
osmolality (>300 mOsm/kg), decreased urine
osmolality (<200 mOsm/kg), and decreased urine
specific gravity (<1.005). Symptoms of hypernatremia
are nonspecific and include decreased level of
consciousness, tremulousness, muscle weakness,
irritability, ataxia, spasticity, confusion,
seizures, coma, and possibly intracranial bleeding
due to increased serum osmolality. Treatment of DI
consists of hydration and hormonal supplementation.
The amount and content of intravenous fluids are
guided by urine volume, serum electrolytes, and
serum osmolality. Free water (H2O)
deficit can be estimated using the following
formula:
|
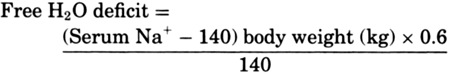 |
If fluid is replaced early, it is not necessary to
administer free water (D5W). Rather, a hypotonic
solution such as 0.45% sodium chloride (NaCl) or
lactated Ringer's may be given. Insulin and
potassium supplementation might be required when
dextrose-containing fluids are used, especially if
corticosteroids are used concomitantly. When
hormonal replacement is required,
1-deamino-8-d-arginine vasopressin (DDAVP), a
synthetic analog of the natural hormone arginine
vasopressin, can be given intravenously,
subcutaneously, orally, or intranasally. The latter
might not be feasible after transnasal
transsphenoidal pituitary surgery. The usual
intravenous or subcutaneous dose is 0.3 mcg/kg/day,
divided and given twice daily. The dose by mouth is
0.05 to 1.2 mg/day, divided and given two to three
times a day. The intranasal dose is 10 to 40 mcg one
to three times a day. The dose is adjusted according
to the patient's sleep pattern and water turnover.
Once intravascular volume has been restored,
persistent hypernatremia may be treated with thiazide diuretics, such as hydrochlorothiazide, 50
to 100 mg/day i.v.
 Rhinorrhea of CSF may develop after transnasal
transsphenoidal operations. Spontaneous resolution
occurs commonly, and clinical observation is
sufficient in most cases. If signs of infection
develop, antibiotic therapy and surgical repair are
indicated.
Rhinorrhea of CSF may develop after transnasal
transsphenoidal operations. Spontaneous resolution
occurs commonly, and clinical observation is
sufficient in most cases. If signs of infection
develop, antibiotic therapy and surgical repair are
indicated.
 Airway obstruction from bleeding and accumulation of
blood and secretions in the pharynx sometimes occurs
after transnasal transsphenoidal surgery. Frequent
assessment of the patency of the airway and adequacy
of ventilation is mandatory. Excessive bleeding
might require reintubation and surgical
consultation.
Airway obstruction from bleeding and accumulation of
blood and secretions in the pharynx sometimes occurs
after transnasal transsphenoidal surgery. Frequent
assessment of the patency of the airway and adequacy
of ventilation is mandatory. Excessive bleeding
might require reintubation and surgical
consultation.
 Postoperative nausea and vomiting might develop due
to intraoperative swallowing of blood during
transsphenoidal resection of pituitary tumors. To
minimize the risk of postoperative nausea and
vomiting, the pharynx and stomach are suctioned at
the conclusion of surgery, and 5HT3 receptor
antagonists are administered prophylactically:
dolasetron, 12.5 mg i.v. in adults and 0.35 mg/kg
i.v. in children; ondansetron, 4 mg i.v. over 4
minutes for adults and children weighing >40 kg and
0.1 mg/kg i.v. over 4 minutes for children weighing
<40 kg, or granisetron, 0.1 to 1 mg i.v.
Postoperative nausea and vomiting might develop due
to intraoperative swallowing of blood during
transsphenoidal resection of pituitary tumors. To
minimize the risk of postoperative nausea and
vomiting, the pharynx and stomach are suctioned at
the conclusion of surgery, and 5HT3 receptor
antagonists are administered prophylactically:
dolasetron, 12.5 mg i.v. in adults and 0.35 mg/kg
i.v. in children; ondansetron, 4 mg i.v. over 4
minutes for adults and children weighing >40 kg and
0.1 mg/kg i.v. over 4 minutes for children weighing
<40 kg, or granisetron, 0.1 to 1 mg i.v.
IV. Complications after operation for head trauma
Systemic sequelae of head trauma frequently become
apparent in the postoperative period. These include
adult respiratory distress syndrome, neurogenic
pulmonary edema (NPE), cardiac arrhythmias,
electrocardiographic (ECG) changes, disseminated
intravascular coagulation, DI, syndrome of
inappropriate antidiuretic hormone secretion
(SIADH), hyperglycemia, nonketotic hyperosmolar
hyperglycemic coma, and gastrointestinal ulcers and
hemorrhage.
 NPE is a fulminant form of pulmonary edema that
progresses rapidly (within hours to days) toward
either resolution or death. The pathologic
characteristics of NPE are marked pulmonary vascular
congestion, pulmonary arteriolar wall rupture,
protein-rich edema fluid, and intra-alveolar
hemorrhage. NPE results from a massive transient
central sympathetic discharge due to an increase in
ICP and is particularly associated with hypothalamic
lesions. The pathophysiology includes systemic
vasoconstriction and left ventricular failure,
redistribution of blood from the systemic to the
pulmonary vessels, pulmonary venous constriction,
and increased pulmonary capillary permeability.
Treatment is aimed at reducing ICP, reducing
sympathetic hyperactivity, mainly by using
alpha-adrenergic blockers such as diazoxide, 1 to 3
mg/kg i.v. every 5 minutes until blood pressure is
controlled, up to 150 mg, or phentolamine, 5 mg i.v.
increments, and providing respiratory supportive
care and inotropic therapy as needed.
NPE is a fulminant form of pulmonary edema that
progresses rapidly (within hours to days) toward
either resolution or death. The pathologic
characteristics of NPE are marked pulmonary vascular
congestion, pulmonary arteriolar wall rupture,
protein-rich edema fluid, and intra-alveolar
hemorrhage. NPE results from a massive transient
central sympathetic discharge due to an increase in
ICP and is particularly associated with hypothalamic
lesions. The pathophysiology includes systemic
vasoconstriction and left ventricular failure,
redistribution of blood from the systemic to the
pulmonary vessels, pulmonary venous constriction,
and increased pulmonary capillary permeability.
Treatment is aimed at reducing ICP, reducing
sympathetic hyperactivity, mainly by using
alpha-adrenergic blockers such as diazoxide, 1 to 3
mg/kg i.v. every 5 minutes until blood pressure is
controlled, up to 150 mg, or phentolamine, 5 mg i.v.
increments, and providing respiratory supportive
care and inotropic therapy as needed.
 The syndrome of inappropriate antidiuretic hormone
(SIADH) causes water retention with continued
urinary excretion of sodium. This leads to
dilutional hyponatremia, decreased serum osmolality,
increased urine osmolality, and decreased urinary
output. Water retention and serum hypo-osmolality
might progress to water intoxication, which leads to
nonspecific symptoms such as nausea, vomiting,
headache, irritability, disorientation, seizures,
and coma. Treatment consists of water restriction,
loop diuretics, and hypertonic saline. In mild
cases, fluid restriction (1 to 1.5 L/day) is
sufficient to correct hyponatremia. Furosemide may
be added because it impairs renal ability to
concentrate urine. Hypertonic saline is usually
reserved for serum sodium of <120 to 125 mEq/L. It
is given in small amounts for a short time (1 to 2
mL/kg/hour for 2 to 3 hours), after which serum
sodium and osmolality are measured. During the acute
phase of SIADH, urine output is measured hourly and
urine osmolality and specific gravity and serum
sodium and osmolality are measured every 6 to 8
hours. Serum sodium should be increased at a rate of
no more than 0.5 mEq/L/hour or 12 mEq/L/day. Faster
rates of correction may cause osmotic demyelination,
which develops over several days. It is associated
with nonspecific signs such as behavioral changes,
movement disorders, seizures, pseudobulbar palsy,
quadriparesis, and coma.
The syndrome of inappropriate antidiuretic hormone
(SIADH) causes water retention with continued
urinary excretion of sodium. This leads to
dilutional hyponatremia, decreased serum osmolality,
increased urine osmolality, and decreased urinary
output. Water retention and serum hypo-osmolality
might progress to water intoxication, which leads to
nonspecific symptoms such as nausea, vomiting,
headache, irritability, disorientation, seizures,
and coma. Treatment consists of water restriction,
loop diuretics, and hypertonic saline. In mild
cases, fluid restriction (1 to 1.5 L/day) is
sufficient to correct hyponatremia. Furosemide may
be added because it impairs renal ability to
concentrate urine. Hypertonic saline is usually
reserved for serum sodium of <120 to 125 mEq/L. It
is given in small amounts for a short time (1 to 2
mL/kg/hour for 2 to 3 hours), after which serum
sodium and osmolality are measured. During the acute
phase of SIADH, urine output is measured hourly and
urine osmolality and specific gravity and serum
sodium and osmolality are measured every 6 to 8
hours. Serum sodium should be increased at a rate of
no more than 0.5 mEq/L/hour or 12 mEq/L/day. Faster
rates of correction may cause osmotic demyelination,
which develops over several days. It is associated
with nonspecific signs such as behavioral changes,
movement disorders, seizures, pseudobulbar palsy,
quadriparesis, and coma.
 Spinal cord injury occurring in conjunction with
head injury might become apparent only in the
postoperative period. Up to 15% of patients with
head injury sustain cervical spine injury as well.
Precautions to avoid exacerbation of spinal cord
injury are continued in the postoperative period
until cervical spine injury is ruled out or
repaired. Pharmacologic therapy to ameliorate spinal
cord injury may be given within 8 hours from injury
in the form of methylprednisolone, loading dose 30
mg/kg i.v.; then 5.4 mg/kg/hour i.v. for 23 hours.
Acute phase spinal shock (usually during the first
week) is treated with fluids, inotropes, and
pressors. During the chronic phase of spinal injury
(after the first week), adequate analgesia is
provided before somatic or splanchnic stimulation in
patients with injury above T6 to avoid the risk of
autonomic hyperreflexia.
Spinal cord injury occurring in conjunction with
head injury might become apparent only in the
postoperative period. Up to 15% of patients with
head injury sustain cervical spine injury as well.
Precautions to avoid exacerbation of spinal cord
injury are continued in the postoperative period
until cervical spine injury is ruled out or
repaired. Pharmacologic therapy to ameliorate spinal
cord injury may be given within 8 hours from injury
in the form of methylprednisolone, loading dose 30
mg/kg i.v.; then 5.4 mg/kg/hour i.v. for 23 hours.
Acute phase spinal shock (usually during the first
week) is treated with fluids, inotropes, and
pressors. During the chronic phase of spinal injury
(after the first week), adequate analgesia is
provided before somatic or splanchnic stimulation in
patients with injury above T6 to avoid the risk of
autonomic hyperreflexia.
 Cardiovascular and respiratory monitoring, aided
with the appropriate imaging and laboratory studies,
is aimed at detecting extracranial injuries and
complications such as pneumothorax, hemothorax,
intra-abdominal or retroperitoneal hemorrhage, and
fat embolism.
Cardiovascular and respiratory monitoring, aided
with the appropriate imaging and laboratory studies,
is aimed at detecting extracranial injuries and
complications such as pneumothorax, hemothorax,
intra-abdominal or retroperitoneal hemorrhage, and
fat embolism.
 Prevention of secondary brain injury is continued in
the postoperative period. Hypotension, hypoxia,
hyperthermia, hyperglycemia, hypoglycemia, increased
ICP, and any aggravating factors such as pain,
nausea, vomiting, seizures, hypertension,
hypercarbia, and impaired cerebral venous drainage
should all be prevented and treated. Conscious,
mechanically ventilated patients are sedated with
short-acting agents, such as propofol, 10 to 30
mcg/kg/minute i.v., or dexmedetomidine, load 1
mcg/kg i.v. over 10 minutes; then 0.2 to 0.7
mcg/kg/hour for <24 hours, to allow intermittent
neurologic assessment. Pain due to the operative
procedure or the primary or associated injury is
relieved with opioids such as morphine, 0.05 mg/kg
i.v., or fentanyl, 0.5 to 1 mcg/kg i.v. Nausea and
vomiting are treated with stomach suctioning (after
ruling out skull base fracture) and pharmacologic
means such as ondansetron, 4 mg i.v.; dolasetron,
12.5 to 25 mg i.v., or granisetron, 0.1 to 1 mg i.v.
Seizure prophylaxis after head trauma is somewhat
controversial. Phenytoin, loading dose 15 mg/kg i.v.
over 20 minutes followed by 5 to 7 mg/kg/day, or
fosphenytoin, loading dose PE 15 to 20 mg/kg i.v.
and then 4 to 6 PE mg/kg/day, may be given for 2
weeks after head injury if there have been no
seizures or longer if there have.
Prevention of secondary brain injury is continued in
the postoperative period. Hypotension, hypoxia,
hyperthermia, hyperglycemia, hypoglycemia, increased
ICP, and any aggravating factors such as pain,
nausea, vomiting, seizures, hypertension,
hypercarbia, and impaired cerebral venous drainage
should all be prevented and treated. Conscious,
mechanically ventilated patients are sedated with
short-acting agents, such as propofol, 10 to 30
mcg/kg/minute i.v., or dexmedetomidine, load 1
mcg/kg i.v. over 10 minutes; then 0.2 to 0.7
mcg/kg/hour for <24 hours, to allow intermittent
neurologic assessment. Pain due to the operative
procedure or the primary or associated injury is
relieved with opioids such as morphine, 0.05 mg/kg
i.v., or fentanyl, 0.5 to 1 mcg/kg i.v. Nausea and
vomiting are treated with stomach suctioning (after
ruling out skull base fracture) and pharmacologic
means such as ondansetron, 4 mg i.v.; dolasetron,
12.5 to 25 mg i.v., or granisetron, 0.1 to 1 mg i.v.
Seizure prophylaxis after head trauma is somewhat
controversial. Phenytoin, loading dose 15 mg/kg i.v.
over 20 minutes followed by 5 to 7 mg/kg/day, or
fosphenytoin, loading dose PE 15 to 20 mg/kg i.v.
and then 4 to 6 PE mg/kg/day, may be given for 2
weeks after head injury if there have been no
seizures or longer if there have.
Clotting may be impaired because of the release of
tissue thromboplastin and a trauma-induced decrease
in platelets, prothrombin (factor II), proaccelerin
(factor V), and plasminogen and increase in fibrin
degradation products.
V. Complications after operation for aneurysm
 Vasospasm. Angiographic narrowing of blood vessels
occurs in approximately 30% of patients between days
4 and 14 after subarachnoid hemorrhage (SAH).
Neurologic dysfunction (disorientation, decreased
level of consciousness, focal deficit) occurs in
approximately 50% of patients who have angiographic
narrowing. The risk for developing vasospasm
correlates with the amount of blood around the
circle of Willis, the preoperative use of
antifibrinolytic therapy, and the postoperative
development of the cerebral salt wasting (CSW)
syndrome. Pharmacologic prophylactic therapy of
vasospasm is initiated within 96 hours of SAH and
consists of nimodipine, 60 mg by mouth every 4 hours
for 21 days or longer. Triple-H therapy
(hypervolemia, hypertension, and hemodilution) may
be used to treat vasospasm following SAH. It
consists of the administration of crystalloid and
colloid solutions to achieve a pulmonary capillary
wedge pressure of 15 mm Hg and hemoglobin level of
11 g/dL and the use of inotropes and vasopressors to
achieve a mean arterial pressure of 120 mm Hg or
more. Antidiuretic therapy with vasopressin is
sometimes necessary to prevent the diuresis induced
by volume loading. Hypotension, heart failure,
myocardial ischemia, and pulmonary edema are
occasional complications of triple-H therapy.
Vasospasm. Angiographic narrowing of blood vessels
occurs in approximately 30% of patients between days
4 and 14 after subarachnoid hemorrhage (SAH).
Neurologic dysfunction (disorientation, decreased
level of consciousness, focal deficit) occurs in
approximately 50% of patients who have angiographic
narrowing. The risk for developing vasospasm
correlates with the amount of blood around the
circle of Willis, the preoperative use of
antifibrinolytic therapy, and the postoperative
development of the cerebral salt wasting (CSW)
syndrome. Pharmacologic prophylactic therapy of
vasospasm is initiated within 96 hours of SAH and
consists of nimodipine, 60 mg by mouth every 4 hours
for 21 days or longer. Triple-H therapy
(hypervolemia, hypertension, and hemodilution) may
be used to treat vasospasm following SAH. It
consists of the administration of crystalloid and
colloid solutions to achieve a pulmonary capillary
wedge pressure of 15 mm Hg and hemoglobin level of
11 g/dL and the use of inotropes and vasopressors to
achieve a mean arterial pressure of 120 mm Hg or
more. Antidiuretic therapy with vasopressin is
sometimes necessary to prevent the diuresis induced
by volume loading. Hypotension, heart failure,
myocardial ischemia, and pulmonary edema are
occasional complications of triple-H therapy.
 Obstructive hydrocephalus due to subarachnoid
blood-induced disturbances in CSF circulation may
occur after SAH. The resulting increase in ICP may
manifest itself as a decreased level of
consciousness. CT scan is diagnostic, and
ventriculostomy with CSF drainage is the effective
therapy.
Obstructive hydrocephalus due to subarachnoid
blood-induced disturbances in CSF circulation may
occur after SAH. The resulting increase in ICP may
manifest itself as a decreased level of
consciousness. CT scan is diagnostic, and
ventriculostomy with CSF drainage is the effective
therapy.
 Hyponatremia after SAH can be due to CSW syndrome
or, less commonly, to SIADH. CSW syndrome is caused
by increased secretion of atrial natriuretic
peptide, brain natriuretic peptide, and C-type
natriuretic peptide. These peptides suppress
aldosterone synthesis and lead to natriuresis,
diuresis, and vasodilatation. Hyponatremia in the
CSW syndrome results from increased renal excretion
of sodium (150 to 200 mEq/L), which is followed by
water with resultant hypovolemia. Hyponatremia of
SIADH is mainly due to water retention in
conjunction with renal excretion of sodium in a
range of 20 to 30 mEq/L. Treatment of the two forms
of hyponatremia is completely different. Patients
with CSW require sodium replacement and fluid
administration, whereas patients with SIADH require
fluid restriction and diuresis. Fluid restriction
and diuresis in a patient with CSW can be fatal due
to the possibility of severe hypovolemia and
cerebral infarction; fluid and salt administered to
a patient with SIADH may lead to osmotic
demyelination. Hypertonic saline may be used with
close monitoring of serum sodium in both CSW
syndrome and SIADH.
Hyponatremia after SAH can be due to CSW syndrome
or, less commonly, to SIADH. CSW syndrome is caused
by increased secretion of atrial natriuretic
peptide, brain natriuretic peptide, and C-type
natriuretic peptide. These peptides suppress
aldosterone synthesis and lead to natriuresis,
diuresis, and vasodilatation. Hyponatremia in the
CSW syndrome results from increased renal excretion
of sodium (150 to 200 mEq/L), which is followed by
water with resultant hypovolemia. Hyponatremia of
SIADH is mainly due to water retention in
conjunction with renal excretion of sodium in a
range of 20 to 30 mEq/L. Treatment of the two forms
of hyponatremia is completely different. Patients
with CSW require sodium replacement and fluid
administration, whereas patients with SIADH require
fluid restriction and diuresis. Fluid restriction
and diuresis in a patient with CSW can be fatal due
to the possibility of severe hypovolemia and
cerebral infarction; fluid and salt administered to
a patient with SIADH may lead to osmotic
demyelination. Hypertonic saline may be used with
close monitoring of serum sodium in both CSW
syndrome and SIADH.
 DI occurs less frequently than CSW
syndrome or SIADH after SAH. Treatment includes hypotonic
fluids in the form of enteral free water or
parenteral D5W, D5 0.2% NaCl, or 0.45% NaCl plus the
administration of DDAVP, 0.3 mcg/kg/day i.v. or
subcutaneously, divided and given twice a day; 0.05
to 1.2 mg/day by mouth; or 10 to 40 mcg one to three
times a day by nasal spray.
DI occurs less frequently than CSW
syndrome or SIADH after SAH. Treatment includes hypotonic
fluids in the form of enteral free water or
parenteral D5W, D5 0.2% NaCl, or 0.45% NaCl plus the
administration of DDAVP, 0.3 mcg/kg/day i.v. or
subcutaneously, divided and given twice a day; 0.05
to 1.2 mg/day by mouth; or 10 to 40 mcg one to three
times a day by nasal spray.
 Intracranial hematomas
might develop at the
operative site or at the bridging dural veins due to
overzealous CSF drainage. Manifestations are those
of increased ICP, which may be associated with focal
deficit. CT scan is diagnostic, and treatment with
surgical evacuation may be required.
Intracranial hematomas
might develop at the
operative site or at the bridging dural veins due to
overzealous CSF drainage. Manifestations are those
of increased ICP, which may be associated with focal
deficit. CT scan is diagnostic, and treatment with
surgical evacuation may be required.
 Seizure prophylaxis is continued in the
postoperative period due to the high risk of
seizures after SAH, especially in hypertensive
patients. Phenytoin is usually given for 3 to 6
months after SAH.
Seizure prophylaxis is continued in the
postoperative period due to the high risk of
seizures after SAH, especially in hypertensive
patients. Phenytoin is usually given for 3 to 6
months after SAH.
 NPE occurs in
some patients after SAH due to the sudden increase
in ICP, which produces intense sympathetic
activation, catecholamine release from the
hypothalamus and the medulla, and increased
pulmonary vascular pressure and permeability. Diagnosis depends on the exclusion of other
causes of pulmonary edema such as triple-H therapy
and aspiration pneumonia. Treatment includes
supplemental oxygen, mechanical ventilation plus
positive end-expiratory pressure, and reduction of
ICP.
NPE occurs in
some patients after SAH due to the sudden increase
in ICP, which produces intense sympathetic
activation, catecholamine release from the
hypothalamus and the medulla, and increased
pulmonary vascular pressure and permeability. Diagnosis depends on the exclusion of other
causes of pulmonary edema such as triple-H therapy
and aspiration pneumonia. Treatment includes
supplemental oxygen, mechanical ventilation plus
positive end-expiratory pressure, and reduction of
ICP.
 After SAH, patients are at moderate risk for
developing deep venous thrombosis (DVT) and
pulmonary embolus (PE). Mechanical DVT prophylaxis,
in the form of graduated stockings or intermittent
pneumatic compression of the lower extremities, is
instituted in all patients after SAH.
Anticoagulation is contraindicated in the acute
postoperative phase. Insertion of an inferior vena
cava filter may be necessary for the prevention of
recurrent pulmonary embolization.
After SAH, patients are at moderate risk for
developing deep venous thrombosis (DVT) and
pulmonary embolus (PE). Mechanical DVT prophylaxis,
in the form of graduated stockings or intermittent
pneumatic compression of the lower extremities, is
instituted in all patients after SAH.
Anticoagulation is contraindicated in the acute
postoperative phase. Insertion of an inferior vena
cava filter may be necessary for the prevention of
recurrent pulmonary embolization.
 Cardiac complications are common after SAH. ECG
changes of arrhythmia, ischemia, or infarction,
which are detected in >50% of patients, occur within
48 hours of SAH but may be first noted in the early
postoperative period. Echocardiography, thallium scintigraphy, and autopsy detect evidence of
myocardial injury. These ECG changes may be due to
hypothalamic injury and high catecholamine levels.
Treatment depends on the severity of the
complications, the hemodynamic stability of the
patient, and concomitant vasospasm. Infarcted,
stunned, or hibernating myocardium might exclude
these patients from triple-H therapy.
Cardiac complications are common after SAH. ECG
changes of arrhythmia, ischemia, or infarction,
which are detected in >50% of patients, occur within
48 hours of SAH but may be first noted in the early
postoperative period. Echocardiography, thallium scintigraphy, and autopsy detect evidence of
myocardial injury. These ECG changes may be due to
hypothalamic injury and high catecholamine levels.
Treatment depends on the severity of the
complications, the hemodynamic stability of the
patient, and concomitant vasospasm. Infarcted,
stunned, or hibernating myocardium might exclude
these patients from triple-H therapy.
VI. Complications after ablation of arteriovenous
malformation (AVM)
After surgery for AVM, patients are at risk of
developing complications similar to those found
after aneurysm surgery (vasospasm, hydrocephalus,
and seizures). In addition, these patients are at
high risk of developing hyperemic complications.
 The syndrome of normal perfusion-pressure
breakthrough or cerebral hyperperfusion is a
hyperemic state characterized by cerebral edema,
swelling, and/or hemorrhage that develops after
resection of AVM. This condition results from the
restoration of cerebral blood flow (CBF) to
chronically hypoperfused areas or from venous
outflow obstruction after AVM ablation. The ensuing
cerebral swelling and hemorrhage cause neurologic
dysfunction and are a major cause of postoperative
morbidity and mortality. Patients who have ischemic
rather than hemorrhagic symptoms preoperatively or
certain angiographic features such as high or
inverse flow in a large, deep, border zone AVM are
particularly at risk. Staged repair and strict
control of blood flow through the AVM may decrease
the risk of hyperemic complications. Treatment of
the manifestations of cerebral hyperemia includes
mechanical hyperventilation, osmotic diuresis, and
barbiturate coma.
The syndrome of normal perfusion-pressure
breakthrough or cerebral hyperperfusion is a
hyperemic state characterized by cerebral edema,
swelling, and/or hemorrhage that develops after
resection of AVM. This condition results from the
restoration of cerebral blood flow (CBF) to
chronically hypoperfused areas or from venous
outflow obstruction after AVM ablation. The ensuing
cerebral swelling and hemorrhage cause neurologic
dysfunction and are a major cause of postoperative
morbidity and mortality. Patients who have ischemic
rather than hemorrhagic symptoms preoperatively or
certain angiographic features such as high or
inverse flow in a large, deep, border zone AVM are
particularly at risk. Staged repair and strict
control of blood flow through the AVM may decrease
the risk of hyperemic complications. Treatment of
the manifestations of cerebral hyperemia includes
mechanical hyperventilation, osmotic diuresis, and
barbiturate coma.
VII. Complications after neuroradiologic procedures
Cerebral vascular embolization procedures may lead
to complications such as SAH, intracerebral
hemorrhage, cerebral ischemia, cerebral infarction,
reperfusion syndrome, seizures, pulmonary embolism,
and contrast reactions. The priority of treatment in
all of these complications is to secure a patent and
protected airway and maintain adequate cerebral
perfusion pressure through the management of blood
pressure and ICP.
 Cerebral hemorrhage may occur either immediately due
to vessel perforation by the catheter or guidewire
or aneurysmal rupture or later due to hyperemic
complications. Most small perforations can be
managed conservatively by observation and follow-up.
Alternatively, the catheter itself can be used to tamponade the source of bleeding and occlude the
perforation. Other treatment measures include
reversal of systemic anticoagulants, seizure
prophylaxis, analgesic and antiemetic therapy, and
insertion of large bore intravenous catheters for
fluid resuscitation and possible blood transfusion.
These measures should be instituted in consultation
with the attending neuroradiologist or neurosurgeon.
Large perforations with massive bleeding require
emergent surgical intervention. However, the
prognosis of extensive bleeding is grave because
most of these patients exsanguinate prior to surgery
owing to the inability to perform craniotomy and
vascular repair in a timely manner, particularly
because most of these vessels are situated deep in
the cerebral parenchyma.
Cerebral hemorrhage may occur either immediately due
to vessel perforation by the catheter or guidewire
or aneurysmal rupture or later due to hyperemic
complications. Most small perforations can be
managed conservatively by observation and follow-up.
Alternatively, the catheter itself can be used to tamponade the source of bleeding and occlude the
perforation. Other treatment measures include
reversal of systemic anticoagulants, seizure
prophylaxis, analgesic and antiemetic therapy, and
insertion of large bore intravenous catheters for
fluid resuscitation and possible blood transfusion.
These measures should be instituted in consultation
with the attending neuroradiologist or neurosurgeon.
Large perforations with massive bleeding require
emergent surgical intervention. However, the
prognosis of extensive bleeding is grave because
most of these patients exsanguinate prior to surgery
owing to the inability to perform craniotomy and
vascular repair in a timely manner, particularly
because most of these vessels are situated deep in
the cerebral parenchyma.
 Cerebral ischemia may occur due to the obstruction
of venous drainage, unintentional occlusion of
surrounding arteries, or local injection of
papaverine for treatment of vasospasm. Cerebral
ischemia/infarction may manifest themselves as
hemiplegia, hemiparesis, cranial nerve palsies,
aphasia, nausea, vomiting, and seizures. Treatment
of cerebral ischemia/infarction in this setting
consists of maintaining adequate cerebral perfusion
pressure by blood pressure and ICP management,
airway protection, and other supportive care as
needed. Intravenous thrombolytic therapy is
contraindicated in this setting. Interventional
neuroradiologic measures to reestablish perfusion
can be attempted.
Cerebral ischemia may occur due to the obstruction
of venous drainage, unintentional occlusion of
surrounding arteries, or local injection of
papaverine for treatment of vasospasm. Cerebral
ischemia/infarction may manifest themselves as
hemiplegia, hemiparesis, cranial nerve palsies,
aphasia, nausea, vomiting, and seizures. Treatment
of cerebral ischemia/infarction in this setting
consists of maintaining adequate cerebral perfusion
pressure by blood pressure and ICP management,
airway protection, and other supportive care as
needed. Intravenous thrombolytic therapy is
contraindicated in this setting. Interventional
neuroradiologic measures to reestablish perfusion
can be attempted.
 Seizures may
occur in patients with preexisting seizure disorders
or in patients with no such history. They may occur
due to cerebral ischemia or infarction, which could
be due to vessel obstruction or trauma, or localized
cerebral edema, which could follow even successful
embolization procedures; or they may be precipitated
by the cerebral hyperperfusion syndrome. Management of seizures should include,
in addition to pharmacologic therapy, identification
and, when possible, treatment of precipitating factor(s), airway management, and stabilization of
hemodynamic parameters. Anticonvulsant therapy
includes midazolam, 0.1 to 0.2 mg/kg i.v.;
lorazepam, 4 to 8 mg i.v.; thiopental, 3 to 5 mg/kg
i.v.; phenytoin, loading dose 10 to 20 mg/kg i.v. no
faster than 50 mg/minute (1,000 mg over 20 minutes
in typical adult) and then 5 to 7 mg/kg/day; or
fosphenytoin, loading dose PE 15 to 20 mg/kg i.v. no
faster than 100 to 150 mg/minute (1,000 mg PE over
10 minutes in typical adult) and then 4 to 6 PE
mg/kg/day.
Seizures may
occur in patients with preexisting seizure disorders
or in patients with no such history. They may occur
due to cerebral ischemia or infarction, which could
be due to vessel obstruction or trauma, or localized
cerebral edema, which could follow even successful
embolization procedures; or they may be precipitated
by the cerebral hyperperfusion syndrome. Management of seizures should include,
in addition to pharmacologic therapy, identification
and, when possible, treatment of precipitating factor(s), airway management, and stabilization of
hemodynamic parameters. Anticonvulsant therapy
includes midazolam, 0.1 to 0.2 mg/kg i.v.;
lorazepam, 4 to 8 mg i.v.; thiopental, 3 to 5 mg/kg
i.v.; phenytoin, loading dose 10 to 20 mg/kg i.v. no
faster than 50 mg/minute (1,000 mg over 20 minutes
in typical adult) and then 5 to 7 mg/kg/day; or
fosphenytoin, loading dose PE 15 to 20 mg/kg i.v. no
faster than 100 to 150 mg/minute (1,000 mg PE over
10 minutes in typical adult) and then 4 to 6 PE
mg/kg/day.
 Pulmonary embolism may occur due to either the
release of embolization materials into the venous
circulation, particularly in AVMs that contain large
fistulae or those of the vein of Galen, or from
vessels accessed en route to the AVM. Depending on
the extent and source of embolism, treatment
consists of securing a patent and protected airway,
maintaining adequate oxygenation and ventilation,
and, in case of cardiopulmonary collapse, performing
surgical embolectomy.
Pulmonary embolism may occur due to either the
release of embolization materials into the venous
circulation, particularly in AVMs that contain large
fistulae or those of the vein of Galen, or from
vessels accessed en route to the AVM. Depending on
the extent and source of embolism, treatment
consists of securing a patent and protected airway,
maintaining adequate oxygenation and ventilation,
and, in case of cardiopulmonary collapse, performing
surgical embolectomy.
 Contrast media reactions may be allergic or nonallergic in nature. Allergic reactions manifest
as generalized flushing, hives, hypotension, and
bronchospasm. Treatment consists of supportive
measures of airway, breathing, and circulation and
pharmacologic therapy in the form of epinephrine,
0.5 to 1 mg i.v. or 3 to 5 mL intratracheally of
1:10,000 solution; hydrocortisone, 100 mg i.v. every
6 hours; and diphenhydramine, 25 to 50 mg i.v. every
4 to 6 hours. Nonallergic reactions occur due to the
sheer volume of the contrast dye, which may lead to
congestive heart failure in susceptible patients or
to osmotic diuresis, which may lead to fluid and
electrolyte imbalances in patients who are already
hypovolemic due to diuretic therapy and/or
restricted salt and water intake.
Contrast media reactions may be allergic or nonallergic in nature. Allergic reactions manifest
as generalized flushing, hives, hypotension, and
bronchospasm. Treatment consists of supportive
measures of airway, breathing, and circulation and
pharmacologic therapy in the form of epinephrine,
0.5 to 1 mg i.v. or 3 to 5 mL intratracheally of
1:10,000 solution; hydrocortisone, 100 mg i.v. every
6 hours; and diphenhydramine, 25 to 50 mg i.v. every
4 to 6 hours. Nonallergic reactions occur due to the
sheer volume of the contrast dye, which may lead to
congestive heart failure in susceptible patients or
to osmotic diuresis, which may lead to fluid and
electrolyte imbalances in patients who are already
hypovolemic due to diuretic therapy and/or
restricted salt and water intake.
 Cerebral hyperperfusion syndrome may occur in areas
surrounding large AVMs due to diversion of blood
flow to the AVM. These hypoperfused areas lose their
ability to autoregulate blood flow and are usually
maximally vasodilated. Following embolization, the
large amount of blood flow flowing through the
erstwhile AVM is now shunted back to these maximally
dilated vessels leading to cerebral hyperemia,
edema, and/or hemorrhage. Methods to prevent this
sudden increase in blood flow include deliberate
hypotension, pretreatment with barbiturates,
clamping of the cervical carotid artery, and staged
embolization of the feeding vessels to allow the
surrounding tissues to regain their autoregulatory
function. Pharmacologic treatment of cerebral edema
includes administration of furosemide, 0.1 to 1
mg/kg i.v., and mannitol, 0.25 to 1 g/kg i.v. over
20 minutes.
Cerebral hyperperfusion syndrome may occur in areas
surrounding large AVMs due to diversion of blood
flow to the AVM. These hypoperfused areas lose their
ability to autoregulate blood flow and are usually
maximally vasodilated. Following embolization, the
large amount of blood flow flowing through the
erstwhile AVM is now shunted back to these maximally
dilated vessels leading to cerebral hyperemia,
edema, and/or hemorrhage. Methods to prevent this
sudden increase in blood flow include deliberate
hypotension, pretreatment with barbiturates,
clamping of the cervical carotid artery, and staged
embolization of the feeding vessels to allow the
surrounding tissues to regain their autoregulatory
function. Pharmacologic treatment of cerebral edema
includes administration of furosemide, 0.1 to 1
mg/kg i.v., and mannitol, 0.25 to 1 g/kg i.v. over
20 minutes.
 Complications due to materials used for
embolization. Glues such as normobutyl cyanoacrylate
(NBCA) and isobutyl 2-cyanoacrylate may cause
pulmonary complications manifesting as hemoptysis
and pleural pain. Particulate materials such as
polyvinyl alcohol (PVA), silicon beads, silk, steel
and platinum microcoils, Gelfoam, and collagen may
cause pulmonary or systemic embolism. Also, PVA is
known to increase the risk of recanalization of the
embolized AVM.
Complications due to materials used for
embolization. Glues such as normobutyl cyanoacrylate
(NBCA) and isobutyl 2-cyanoacrylate may cause
pulmonary complications manifesting as hemoptysis
and pleural pain. Particulate materials such as
polyvinyl alcohol (PVA), silicon beads, silk, steel
and platinum microcoils, Gelfoam, and collagen may
cause pulmonary or systemic embolism. Also, PVA is
known to increase the risk of recanalization of the
embolized AVM.
VIII. Complications after carotid endarterectomy
(CEA)
 Cardiac ischemia and infarction are the leading
cause of mortality after CEA. Coronary artery
disease is common among patients undergoing CEA.
Perioperative tachycardia, hypertension, and
hypotension increase the risk of perioperative
myocardial ischemia and infarction. The
alpha-agonists, such as phenylephrine, are
preferable in the treatment of hypotension in this
setting because they raise blood pressure without
significantly increasing heart rate. However, the
ensuing hypertension may be detrimental. Combined
alpha- and beta-agonists such as ephedrine increase
heart rate and have been associated with myocardial
ischemia and infarction in this setting.
Cardiac ischemia and infarction are the leading
cause of mortality after CEA. Coronary artery
disease is common among patients undergoing CEA.
Perioperative tachycardia, hypertension, and
hypotension increase the risk of perioperative
myocardial ischemia and infarction. The
alpha-agonists, such as phenylephrine, are
preferable in the treatment of hypotension in this
setting because they raise blood pressure without
significantly increasing heart rate. However, the
ensuing hypertension may be detrimental. Combined
alpha- and beta-agonists such as ephedrine increase
heart rate and have been associated with myocardial
ischemia and infarction in this setting.
 Occlusion of the operated carotid artery should be
suspected whenever new neurologic symptoms develop
postoperatively. This is one cause of postoperative
cerebral ischemia that is amenable to surgical
intervention. Early diagnosis and treatment
significantly alter outcome. A Doppler flow study
can detect cessation of flow in the involved vessel,
and angiography can confirm vascular occlusion.
Surgical reexploration need not await angiographic
confirmation but may be undertaken on the basis of
the clinical picture and the ultrasound examination.
Occlusion of the operated carotid artery should be
suspected whenever new neurologic symptoms develop
postoperatively. This is one cause of postoperative
cerebral ischemia that is amenable to surgical
intervention. Early diagnosis and treatment
significantly alter outcome. A Doppler flow study
can detect cessation of flow in the involved vessel,
and angiography can confirm vascular occlusion.
Surgical reexploration need not await angiographic
confirmation but may be undertaken on the basis of
the clinical picture and the ultrasound examination.
 The cerebral hyperperfusion syndrome may develop
after CEA due to the sudden increase in CBF in a
maximally dilated vascular bed that has lost its
ability to autoregulate because of longstanding
hypoperfusion. This hyperperfusion may lead to
cerebral edema or hemorrhage with headache,
seizures, decreased level of consciousness, and
focal neurologic deficit. Severe carotid stenosis
and hypertension contribute to the development of
this syndrome. Careful control of blood pressure is
essential in preventing hyperperfusion. Mild
elevation of blood pressure need not be treated in
the postoperative period, whereas moderate to severe
hypertension should be reduced to avoid the cerebral
hyperperfusion syndrome. Titratable, short-acting
agents such as sodium nitroprusside (SNP), 0.25 to 8
mcg/kg/minute i.v., and esmolol, loading dose 500
mcg/kg over 1 minute followed by 50 to 300
mcg/kg/minute i.v., are preferable in this setting.
The beta-blocking effects of esmolol offset the
sympathetic hyperactivity from SNP.
The cerebral hyperperfusion syndrome may develop
after CEA due to the sudden increase in CBF in a
maximally dilated vascular bed that has lost its
ability to autoregulate because of longstanding
hypoperfusion. This hyperperfusion may lead to
cerebral edema or hemorrhage with headache,
seizures, decreased level of consciousness, and
focal neurologic deficit. Severe carotid stenosis
and hypertension contribute to the development of
this syndrome. Careful control of blood pressure is
essential in preventing hyperperfusion. Mild
elevation of blood pressure need not be treated in
the postoperative period, whereas moderate to severe
hypertension should be reduced to avoid the cerebral
hyperperfusion syndrome. Titratable, short-acting
agents such as sodium nitroprusside (SNP), 0.25 to 8
mcg/kg/minute i.v., and esmolol, loading dose 500
mcg/kg over 1 minute followed by 50 to 300
mcg/kg/minute i.v., are preferable in this setting.
The beta-blocking effects of esmolol offset the
sympathetic hyperactivity from SNP.
 Hypotension is poorly tolerated by hypertensive
patients who have a rightward shift in their
autoregulatory curve. Hypotension can lead to
cerebral and cardiac hypoperfusion and can increase
the risk of thrombus formation in the operated
vessel. Blood pressure is usually kept at 20% above
baseline. Hypotension is treated with volume
expansion and infusion of a short-acting
alpha-agonist such as phenylephrine, 40 to 180
mcg/minute i.v., mixed as 20 mg in 250 mL D5W at 30
to 160 mL/hour.
Hypotension is poorly tolerated by hypertensive
patients who have a rightward shift in their
autoregulatory curve. Hypotension can lead to
cerebral and cardiac hypoperfusion and can increase
the risk of thrombus formation in the operated
vessel. Blood pressure is usually kept at 20% above
baseline. Hypotension is treated with volume
expansion and infusion of a short-acting
alpha-agonist such as phenylephrine, 40 to 180
mcg/minute i.v., mixed as 20 mg in 250 mL D5W at 30
to 160 mL/hour.
 Treatment of stroke after CEA consists of blood
pressure management, supportive care, and treatment
of complications. Intravenous thrombolytic therapy
is contraindicated in the postoperative period.
Intra-arterial thrombolytic therapy may be
considered in institutions that have the expertise.
Neuroprotective therapy, still the subject of
clinical trials, has not been approved for clinical
use.
Treatment of stroke after CEA consists of blood
pressure management, supportive care, and treatment
of complications. Intravenous thrombolytic therapy
is contraindicated in the postoperative period.
Intra-arterial thrombolytic therapy may be
considered in institutions that have the expertise.
Neuroprotective therapy, still the subject of
clinical trials, has not been approved for clinical
use.
 Airway obstruction can result from hematoma
formation and can be aggravated by laryngeal edema
and cranial nerve injury. Reestablishing airway
patency might require suture removal and drainage of
the hematoma. This is best accomplished by a surgeon
in conjunction with tracheal intubation and racemic
epinephrine. If the patient is in extremis, the
first person to reach the bedside opens the wound to
secure the airway.
Airway obstruction can result from hematoma
formation and can be aggravated by laryngeal edema
and cranial nerve injury. Reestablishing airway
patency might require suture removal and drainage of
the hematoma. This is best accomplished by a surgeon
in conjunction with tracheal intubation and racemic
epinephrine. If the patient is in extremis, the
first person to reach the bedside opens the wound to
secure the airway.
IX. Complications after vertebral column procedures
 Complications after anterior cervical discectomy.
The patient's trachea is usually extubated in the
operating room after an uncomplicated discectomy.
However, tracheal intubation may be maintained
postoperatively if upper airway edema is anticipated
after a prolonged operation or one associated with
infusion of large volumes of fluid. It is important
to prevent the patient from coughing and straining
while the trachea is intubated. This may cause the
newly placed bone graft to dislodge, which might
compress the trachea or the esophagus and require
reoperation. After extubation, the patient's voice
is evaluated to detect recurrent laryngeal nerve
injury, a benign complication that usually resolves
over days to weeks.
Complications after anterior cervical discectomy.
The patient's trachea is usually extubated in the
operating room after an uncomplicated discectomy.
However, tracheal intubation may be maintained
postoperatively if upper airway edema is anticipated
after a prolonged operation or one associated with
infusion of large volumes of fluid. It is important
to prevent the patient from coughing and straining
while the trachea is intubated. This may cause the
newly placed bone graft to dislodge, which might
compress the trachea or the esophagus and require
reoperation. After extubation, the patient's voice
is evaluated to detect recurrent laryngeal nerve
injury, a benign complication that usually resolves
over days to weeks.
 Complications after cervical corpectomy and
stabilization. These procedures are usually more
invasive, more prolonged, associated with more fluid
administration, and therefore more likely to cause
airway edema at the conclusion of surgery. The
patient's trachea usually remains intubated until
the airway edema resolves, as evidenced by the
ability of the patient to breathe around the
endotracheal tube after the cuff has been deflated.
Sedation is provided as needed while the trachea is
intubated.
Complications after cervical corpectomy and
stabilization. These procedures are usually more
invasive, more prolonged, associated with more fluid
administration, and therefore more likely to cause
airway edema at the conclusion of surgery. The
patient's trachea usually remains intubated until
the airway edema resolves, as evidenced by the
ability of the patient to breathe around the
endotracheal tube after the cuff has been deflated.
Sedation is provided as needed while the trachea is
intubated.
 Complications after transoral resection of the
odontoid and occipitocervical fusion. This operation
is usually performed in two steps: anterior transoral resection of the odontoid and posterior
occipitocervical fusion. Airway management involves
either tracheotomy or an oral endotracheal tube
draped out of the surgical field as for
tonsillectomy. The procedure is associated with
significant posterior pharyngeal swelling, which
requires postoperative intubation for several days.
The patient is usually awakened at the conclusion of
surgery to undergo a neurologic examination and then
sedated again. The degree of resolution of the
airway edema is evaluated by deflating the cuff of
the endotracheal tube and establishing the ability
of the patient to breathe around the endotracheal
tube.
Complications after transoral resection of the
odontoid and occipitocervical fusion. This operation
is usually performed in two steps: anterior transoral resection of the odontoid and posterior
occipitocervical fusion. Airway management involves
either tracheotomy or an oral endotracheal tube
draped out of the surgical field as for
tonsillectomy. The procedure is associated with
significant posterior pharyngeal swelling, which
requires postoperative intubation for several days.
The patient is usually awakened at the conclusion of
surgery to undergo a neurologic examination and then
sedated again. The degree of resolution of the
airway edema is evaluated by deflating the cuff of
the endotracheal tube and establishing the ability
of the patient to breathe around the endotracheal
tube.
 Complications after posterior cervical spine
procedures. Complications are related to the
patient's intraoperative position and the degree of
airway edema and respiratory dysfunction at the
conclusion of surgery.
Complications after posterior cervical spine
procedures. Complications are related to the
patient's intraoperative position and the degree of
airway edema and respiratory dysfunction at the
conclusion of surgery.
 The operative positions include prone, sitting, and
three-quarters prone. Complications of the prone
position include injury at pressure points: eyes,
cheeks, lips, breasts, and genitalia. Injury to
these structures requires appropriate surgical
consultation.
The operative positions include prone, sitting, and
three-quarters prone. Complications of the prone
position include injury at pressure points: eyes,
cheeks, lips, breasts, and genitalia. Injury to
these structures requires appropriate surgical
consultation.
 Airway edema depends on the duration of surgery, the
amount of blood loss, and fluid administration.
Airway edema depends on the duration of surgery, the
amount of blood loss, and fluid administration.
 Respiratory dysfunction may exist preoperatively due
to involvement of C3-C5 nerve roots or may result
from resection of intramedullary spinal cord tumors.
These patients are evaluated before extubation to
demonstrate the patency of the airway (lack of
airway edema) and adequacy of respiratory function
(tidal volume, vital capacity, negative inspiratory
force). Postoperative intubation and mechanical
ventilation might be required until airway edema
resolves and respiratory function recovers.
Respiratory dysfunction may exist preoperatively due
to involvement of C3-C5 nerve roots or may result
from resection of intramedullary spinal cord tumors.
These patients are evaluated before extubation to
demonstrate the patency of the airway (lack of
airway edema) and adequacy of respiratory function
(tidal volume, vital capacity, negative inspiratory
force). Postoperative intubation and mechanical
ventilation might be required until airway edema
resolves and respiratory function recovers.
 Complications after scoliosis surgery. These
procedures are performed with the patient in the
prone or lateral position or a combination of a
lateral-position operation followed immediately or 1
to 2 weeks later by a prone-position operation.
Complications after scoliosis surgery. These
procedures are performed with the patient in the
prone or lateral position or a combination of a
lateral-position operation followed immediately or 1
to 2 weeks later by a prone-position operation.
 Complications related to the prone position are
those of pressure point injury, particularly the
eyes. Ischemic optic neuropathy (ION) is correlated
with intraoperative hypotension and ischemia,
regardless of position. Central retinal artery
occlusion can occur during prone-position procedures
due to improper protection of the eyes. Scoliosis
surgery patients are therefore particularly
vulnerable to ION, which is manifest postoperatively
by varying degrees of unilateral or bilateral
decreases in visual acuity or defects in the visual
field. The decrease in visual acuity may resolve
over time, but the defects in the visual field
usually persist. Postoperative visual examination is
performed routinely in these patients;
ophthalmologic consultation is requested if any
abnormality is detected.
Complications related to the prone position are
those of pressure point injury, particularly the
eyes. Ischemic optic neuropathy (ION) is correlated
with intraoperative hypotension and ischemia,
regardless of position. Central retinal artery
occlusion can occur during prone-position procedures
due to improper protection of the eyes. Scoliosis
surgery patients are therefore particularly
vulnerable to ION, which is manifest postoperatively
by varying degrees of unilateral or bilateral
decreases in visual acuity or defects in the visual
field. The decrease in visual acuity may resolve
over time, but the defects in the visual field
usually persist. Postoperative visual examination is
performed routinely in these patients;
ophthalmologic consultation is requested if any
abnormality is detected.
 Patients with scoliosis may have respiratory
dysfunction preoperatively due to skeletal
deformities, muscular weakness, central nervous
system (CNS) dysfunction, or a combination of
factors. This respiratory dysfunction might be
aggravated postoperatively by the residual effect of
anesthetics, inadequate reversal of muscle relaxants
due to hypothermia, restrictive effect of pain, and
pneumothorax. Airway edema from positioning,
prolonged surgery, and administration of a large
volume of fluids contributes to the respiratory
compromise. Extubation of the trachea is undertaken
only after airway patency and adequacy of
respiratory function have been established. Pain
control is essential for patient comfort and the
maintenance of adequate respiratory function.
Patients with scoliosis may have respiratory
dysfunction preoperatively due to skeletal
deformities, muscular weakness, central nervous
system (CNS) dysfunction, or a combination of
factors. This respiratory dysfunction might be
aggravated postoperatively by the residual effect of
anesthetics, inadequate reversal of muscle relaxants
due to hypothermia, restrictive effect of pain, and
pneumothorax. Airway edema from positioning,
prolonged surgery, and administration of a large
volume of fluids contributes to the respiratory
compromise. Extubation of the trachea is undertaken
only after airway patency and adequacy of
respiratory function have been established. Pain
control is essential for patient comfort and the
maintenance of adequate respiratory function.
 Postoperative hemorrhage can continue either
externally from surgical drains or internally into
the operative site. Monitoring of systemic blood
pressure, urinary output, central venous pressure
(if available), hemoglobin, and hematocrit is
necessary. Excessive blood loss is treated with
volume expansion, blood transfusion, hemodynamic
support, and surgical consultation.
Postoperative hemorrhage can continue either
externally from surgical drains or internally into
the operative site. Monitoring of systemic blood
pressure, urinary output, central venous pressure
(if available), hemoglobin, and hematocrit is
necessary. Excessive blood loss is treated with
volume expansion, blood transfusion, hemodynamic
support, and surgical consultation.
 Central and peripheral neurologic function is
monitored postoperatively. Patients might develop
spinal cord injury due to instrumentation or
hematoma formation and CNS dysfunction due to
pharmacologic or hemodynamic factors. Neurologic
dysfunction should prompt a thorough examination of
the patient, review of medication, hemodynamic and
laboratory workup, surgical consultation, and
supportive therapy as needed.
Central and peripheral neurologic function is
monitored postoperatively. Patients might develop
spinal cord injury due to instrumentation or
hematoma formation and CNS dysfunction due to
pharmacologic or hemodynamic factors. Neurologic
dysfunction should prompt a thorough examination of
the patient, review of medication, hemodynamic and
laboratory workup, surgical consultation, and
supportive therapy as needed.
 Complications after lateral extracavitary and
percutaneous endoscopic procedures. A factor common
to these two operations is the intraoperative use of
double-lumen endotracheal tubes or other forms of
one-lung ventilation. Pulmonary edema may develop
with reexpansion of the lung after one-lung
ventilation for >3 hours, most likely within 1 hour
of reexpansion. If mechanical ventilation is to be
maintained in the postoperative period, the
double-lumen tube is exchanged for a single-lumen
one. Alternatively, a univent tube, which is a
single-lumen tube with a movable endobronchial
blocker, may be used intraoperatively and
postoperatively during weaning and until extubation.
Complications after lateral extracavitary and
percutaneous endoscopic procedures. A factor common
to these two operations is the intraoperative use of
double-lumen endotracheal tubes or other forms of
one-lung ventilation. Pulmonary edema may develop
with reexpansion of the lung after one-lung
ventilation for >3 hours, most likely within 1 hour
of reexpansion. If mechanical ventilation is to be
maintained in the postoperative period, the
double-lumen tube is exchanged for a single-lumen
one. Alternatively, a univent tube, which is a
single-lumen tube with a movable endobronchial
blocker, may be used intraoperatively and
postoperatively during weaning and until extubation.
X. Complications after spinal cord procedures
 Complications after syringomyelia repair.
Respiratory complications are the main concern.
Preoperative respiratory dysfunction is due to
skeletal deformities, autonomic dysfunction, and
chronic aspiration (from depressed gag reflex and
vocal cord paralysis). This respiratory dysfunction
may be exacerbated in the postoperative period by
airway edema, residual anesthetic effects, and
incomplete reversal of muscle relaxants, which could
be aggravated by the hypothermia of autonomic
dysfunction. Extubation is performed only after the
adequacy of respiratory function and airway patency
have been established. Respiratory monitoring and
support are maintained in the postoperative period
as needed.
Complications after syringomyelia repair.
Respiratory complications are the main concern.
Preoperative respiratory dysfunction is due to
skeletal deformities, autonomic dysfunction, and
chronic aspiration (from depressed gag reflex and
vocal cord paralysis). This respiratory dysfunction
may be exacerbated in the postoperative period by
airway edema, residual anesthetic effects, and
incomplete reversal of muscle relaxants, which could
be aggravated by the hypothermia of autonomic
dysfunction. Extubation is performed only after the
adequacy of respiratory function and airway patency
have been established. Respiratory monitoring and
support are maintained in the postoperative period
as needed.
 Complications after resection of spinal cord tumors.
Significant cord edema may develop up to 24 hours
after resection. The edema-induced respiratory
dysfunction associated with the resection of upper
cervical cord tumors might not become apparent in
the immediate postoperative period. Respiratory
monitoring of these patients in an intensive care
setting for at least 24 hours postoperatively is
therefore necessary.
Complications after resection of spinal cord tumors.
Significant cord edema may develop up to 24 hours
after resection. The edema-induced respiratory
dysfunction associated with the resection of upper
cervical cord tumors might not become apparent in
the immediate postoperative period. Respiratory
monitoring of these patients in an intensive care
setting for at least 24 hours postoperatively is
therefore necessary.
 Complications after spinal cord injury. Patients
with spinal cord injury develop complications
related to sympathectomy, skeletal muscle
denervation, immobilization, chronic
instrumentation, decreased respiratory force, and
coexisting injuries involving other organs.
Approximately 50% of patients with cervical spine
injury have concurrent head trauma and approximately
25% have injuries to the chest, abdomen, or
extremities.
Complications after spinal cord injury. Patients
with spinal cord injury develop complications
related to sympathectomy, skeletal muscle
denervation, immobilization, chronic
instrumentation, decreased respiratory force, and
coexisting injuries involving other organs.
Approximately 50% of patients with cervical spine
injury have concurrent head trauma and approximately
25% have injuries to the chest, abdomen, or
extremities.
 The severity and mechanism of the initial spinal
shock, which lasts for 2 to 3 weeks after injury,
are related to the level of the injury. With
mid-thoracic lesions (T6-7), hypotension may not be
severe and is mainly due to vasodilatation. With
higher lesions (T4 or above), hypotension may be
profound when vasodilatation is added to a decrease
in heart rate, contractility, and compliance from
loss of cardiac accelerator fibers (T1-4).
The severity and mechanism of the initial spinal
shock, which lasts for 2 to 3 weeks after injury,
are related to the level of the injury. With
mid-thoracic lesions (T6-7), hypotension may not be
severe and is mainly due to vasodilatation. With
higher lesions (T4 or above), hypotension may be
profound when vasodilatation is added to a decrease
in heart rate, contractility, and compliance from
loss of cardiac accelerator fibers (T1-4).
 Succinylcholine may cause hyperkalemia in patients
who have denervated muscle because of the increased
number and sensitivity of their neuromuscular
cholinergic receptors. These receptor changes start
3 days after the injury and persist for 6 to 8
months. Succinylcholine is therefore avoided in
spinal cord injury patients from 48 hours to 8
months after the injury.
Succinylcholine may cause hyperkalemia in patients
who have denervated muscle because of the increased
number and sensitivity of their neuromuscular
cholinergic receptors. These receptor changes start
3 days after the injury and persist for 6 to 8
months. Succinylcholine is therefore avoided in
spinal cord injury patients from 48 hours to 8
months after the injury.
 Autonomic hyperreflexia is caused by noxious
stimulation below the level of the lesion in a
patient with a sympathectomy at or above T6.
Autonomic hyperreflexia is caused by noxious
stimulation below the level of the lesion in a
patient with a sympathectomy at or above T6.
1.
The risk of autonomic hyperreflexia is highest
during the fourth week after injury but continues
thereafter. At this time, while the patient is
recovering from the spinal shock phase, which lasts
for 2 to 3 weeks after the initial injury, the
flaccid paralysis changes to spastic paralysis
because of the absence of the effect of central
inhibitory pathways. The efferent sympathetic fibers
recover from the initial injury but remain
unaffected by central inhibitory input from the
brain stem and hypothalamus.
2.
The severity and manifestations of autonomic
hyperreflexia are affected by the level of the
sympathectomy. With mid-thoracic lesions below the
level of cardiac accelerator fibers, hypertension is
accompanied by reflex bradycardia transmitted via
cardiac accelerator fibers and the vagus. In
patients whose sympathectomy is above the level of
the thoracic cardiac accelerator fibers, tachycardia
may occur because cardiac accelerator fibers become
part of the efferent sympathetic activity rather
than part of the central inhibitory input from the
brain stem and hypothalamus. Arrhythmias and
occasionally heart block may accompany changes in
heart rate. Clinical manifestations of autonomic
hyperreflexia include vasodilatation, decreased
sympathetic activity, and increased vagal activity
above the level of the lesion such as nasal
congestion, flushing, headache, dyspnea, nausea, and
visceral muscle contraction. Vasoconstriction and
increased sympathetic activity below the level of
the lesion cause vasoconstrictive pallor, sweating,
piloerection, and somatic muscle fasciculation.
Patients also develop hypertension with headache,
blurred vision, myocardial infarction, and retinal,
subarachnoid, and cerebral hemorrhages that may lead
to syncope, convulsions, and death.
3.
Autonomic hyperreflexia may be prevented or
attenuated by regional or deep general anesthesia,
but this is usually impractical in the
postanesthesia care unit. Pharmacologic means of
preventing and treating autonomic hyperreflexia
include alpha-blockers such as diazoxide, 1 to 3
mg/kg i.v. every 5 minutes up to 150 mg, or
phentolamine, 5 mg i.v. increments, vasodilators
such as SNP, 0.25 to 8 mcg/kg/minute i.v., and
selective beta-blockers such as esmolol, loading
dose 500 mcg/kg followed by 50 to 300 mcg/kg/minute
i.v., for supraventricular tachycardia.
|