|
| Head Injury |
Supratentorial Tumors |
Posterior Fossa Surgery|
Intracranial Aneurysms
|Ischemic Cerebrovascular Diseases
|Neuroendocrine Tumors
|Epilepsy-Awake
Craniotomy-Intraoperative MRI
|Spinal Cord Injury and Procedures
|Pediatric Neuroanesthesia
|Neurosurgery in the Pregnant
Patient
|Management of Therapeutic
Interventional Neuroradiology
|Management in Diagnostic
Neuroradiology
|

Neuroendocrine
Tumors: Pathophysiology
I. Anatomy
 Intracranial mass. Many
neuroendocrine tumors are microadenomas (<10 mm in
size) and require routine induction and maintenance
of anesthesia for their surgical extirpation. Some
tumors are very large and require attention to blood
pressure (BP), airway dynamics, and other factors
affecting intracranial pressure (ICP) during
induction of anesthesia, maintenance, and emergence.
Intracranial mass. Many
neuroendocrine tumors are microadenomas (<10 mm in
size) and require routine induction and maintenance
of anesthesia for their surgical extirpation. Some
tumors are very large and require attention to blood
pressure (BP), airway dynamics, and other factors
affecting intracranial pressure (ICP) during
induction of anesthesia, maintenance, and emergence.
 Pituitary gland and stalk
are very close to the optic chiasm, intracranial
carotid arteries, cavernous sinuses, and cranial
nerves.
Pituitary gland and stalk
are very close to the optic chiasm, intracranial
carotid arteries, cavernous sinuses, and cranial
nerves.
 Pituitary adenomas may
be either intrasellar or extracranial and may extend
laterally into the cavernous sinuses.
Pituitary adenomas may
be either intrasellar or extracranial and may extend
laterally into the cavernous sinuses.
 Cavernous sinus contains the intrasphenoid carotid
artery and cranial nerves III, IV, V1, V2, and VI
(Figure-1).
Cavernous sinus contains the intrasphenoid carotid
artery and cranial nerves III, IV, V1, V2, and VI
(Figure-1).
 Magnetic resonance imaging (MRI) appearance and
extension of the tumor are important for planning
anesthetic management and neurophysiologic
monitoring.
Magnetic resonance imaging (MRI) appearance and
extension of the tumor are important for planning
anesthetic management and neurophysiologic
monitoring.
 Pituitary tumors
can extend out of the sella into the intracranial
space and involve the optic chiasm and carotid
arteries.
Pituitary tumors
can extend out of the sella into the intracranial
space and involve the optic chiasm and carotid
arteries.
II. Endocrine
physiology: anterior pituitary
 The hypothalamus secretes hormone-releasing factor
(RF), which is transported to the median eminence of
the hypothalamus by axonal flow. The RF is then
transported via the hypothalamo-hypophyseal portal
system to the anterior pituitary. Hormones are
secreted into the systemic circulation in response
to a variety of stimuli. Target organs respond to
stimuli and send negative feedback to the pituitary
and the hypothalamus to turn off the secretion of RF
and stop the release of the hormone. The incidence
of asymptomatic microadenomas on autopsy is up to
27%. The peak age of occurrence is 40 years.
Prolactinomas account for approximately 40% of all
symptomatic microadenomas.
The hypothalamus secretes hormone-releasing factor
(RF), which is transported to the median eminence of
the hypothalamus by axonal flow. The RF is then
transported via the hypothalamo-hypophyseal portal
system to the anterior pituitary. Hormones are
secreted into the systemic circulation in response
to a variety of stimuli. Target organs respond to
stimuli and send negative feedback to the pituitary
and the hypothalamus to turn off the secretion of RF
and stop the release of the hormone. The incidence
of asymptomatic microadenomas on autopsy is up to
27%. The peak age of occurrence is 40 years.
Prolactinomas account for approximately 40% of all
symptomatic microadenomas.
 Anterior pituitary hormones (adrenocorticotropic
hormone [ACTH], growth hormone [GH], prolactin
[PRL], thyroid-stimulating hormone [TSH],
luteinizing hormone [LH], melanocyte-stimulating
hormone [MSH], and follicle-stimulating hormone
[FSH]) may all be produced by microadenomas. ACTH,
GH, and PRL are produced most commonly. All have
inhibiting factors and RFs, and all can be
accurately measured in the blood using
radioimmunoassay techniques.
Anterior pituitary hormones (adrenocorticotropic
hormone [ACTH], growth hormone [GH], prolactin
[PRL], thyroid-stimulating hormone [TSH],
luteinizing hormone [LH], melanocyte-stimulating
hormone [MSH], and follicle-stimulating hormone
[FSH]) may all be produced by microadenomas. ACTH,
GH, and PRL are produced most commonly. All have
inhibiting factors and RFs, and all can be
accurately measured in the blood using
radioimmunoassay techniques.
|
Figure-1. Coronal section through pituitary gland
demonstrating perisellar structures and sphenoid
sinuses. Cranial nerves: ocular nerve (III),
trochlear nerve (IV), trigeminal nerve (V), abducens
nerve (VI). |
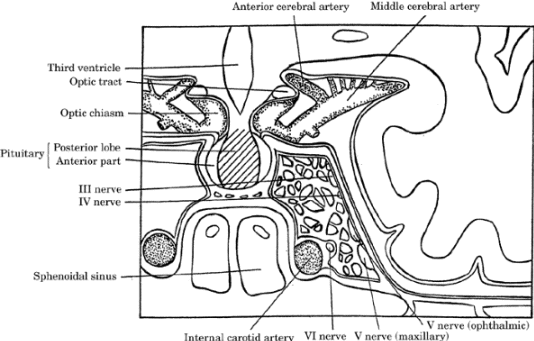 |
 Cushing's disease is caused by an ACTH-secreting
pituitary tumor.
Cushing's disease is caused by an ACTH-secreting
pituitary tumor.
 Cushing's syndrome occurs when there is an
ACTH-dependent (ACTH administration, ectopic ACTH
syndrome) or ACTH-independent (adrenal adenoma,
carcinoma, or exogenous cortisol administration)
excess secretion of cortisol.
Cushing's syndrome occurs when there is an
ACTH-dependent (ACTH administration, ectopic ACTH
syndrome) or ACTH-independent (adrenal adenoma,
carcinoma, or exogenous cortisol administration)
excess secretion of cortisol.
 Ectopic ACTH is associated with a primary oat cell
carcinoma of the lung.
Ectopic ACTH is associated with a primary oat cell
carcinoma of the lung.
a.
The tumor produces ACTH- and corticotropin-releasing
factor (CRF)-like peptides.
b.
Plasma cortisol is >50 mcg/dL.
c.
Explosive hypercortisolism may occur with the
following:
(1) Hypertension
(2) Glucose intolerance
(3) Hyperaldosteronism (hypokalemic alkalosis)
(4) Marked hyperpigmentation
 Cushing's disease
Cushing's disease
1. Truncal obesity, posterior cervical fat pads,
osteoporosis, and moon facies are characteristics.
2. Hypertension and glucose intolerance can occur.
3. Adrenal hyperplasia. In the past, many patients had
bilateral adrenalectomy to treat what was thought to
be a primary adrenal condition (Nelson's syndrome).
These patients may then come for surgery later to
remove what was actually a primary pituitary tumor.
4. CRF is stimulated by acetylcholine and serotonin and
inhibited by norepinephrine.
5. Cortisol secretion is 16 mcg/day, 75% of which is
bound to transcortin protein.
6. Normal diurnal variation is 4 am to 8 am: 25 mcg/dL;
4 pm to 8 pm: <10 mcg/dL. Normal diurnal variation
increases with stress and pregnancy.
7. Diagnosis is based on loss of diurnal variation,
increased ACTH, and probable MRI evidence of sellar
adenoma.
 Acromegaly, a GH-producing tumor, leads to the
following:
Acromegaly, a GH-producing tumor, leads to the
following:
 Signs and symptoms
Signs and symptoms
1.
Bony and soft tissue enlargement (frontal bossing,
prognathism, increased ring, glove, and shoe size)
2.
Hypertension
3.
Glucose intolerance
4.
Visual loss if tumor is large and involves chiasm
5.
Hoarseness (soft tissue stretching of cranial nerve
X)
6.
Dyspnea (narrow glottis from soft tissue overgrowth)
7.
Cardiomyopathy from lymphocytic infiltration, a
common cause of death, if untreated
8.
Carpal tunnel syndrome from soft tissue overgrowth
9.
Lumbar spinal stenosis and cervical compression from
bony overgrowth
 Hypoglycemia is the most potent stimulus to the
secretion of GH. Somatostatin inhibits the release
of GH.
Hypoglycemia is the most potent stimulus to the
secretion of GH. Somatostatin inhibits the release
of GH.
 Diagnosis includes random blood GH of >10 ng/mL (2
to 5 ng/mL normally) and elevation of somatomedin C
(produced in the liver in response to GH stimulation
so that high levels occur only with acromegaly).
Diagnosis includes random blood GH of >10 ng/mL (2
to 5 ng/mL normally) and elevation of somatomedin C
(produced in the liver in response to GH stimulation
so that high levels occur only with acromegaly).
 Acromegaly and the airway
Acromegaly and the airway
1.
Hypertrophy of the mandible, nasal turbinates, soft
palate, tonsils, epiglottis, arytenoids, tongue,
lips, and nose may occur.
2.
The glottis might be narrow.
3.
Vocal cord paralysis can be present from soft tissue
overgrowth.
4.
Most often routine intubation techniques are
successful, but the ready availability of extra-long
blades, smaller endotracheal tubes, laryngeal masks,
and fiberoptic intubation equipment is essential.
5.
Anticipate difficult mask fit and potential
postextubation stridor.
6.
Patients frequently have a consultation with an
otolaryngologist before an operation. A report of
the indirect laryngoscopy performed by that
consultant is obtained to facilitate anticipation of
and preparation for airway difficulty.
 PRL-secreting tumors may be larger in men than women
because women tend to seek medical attention earlier
because of infertility. Other signs and symptoms
include amenorrhea, galactorrhea, anovulation,
decreased libido, gynecomastia, and osteoporosis.
PRL-secreting tumors may be larger in men than women
because women tend to seek medical attention earlier
because of infertility. Other signs and symptoms
include amenorrhea, galactorrhea, anovulation,
decreased libido, gynecomastia, and osteoporosis.
 PRL secretion is primarily regulated by dopamine,
which functions as an inhibitory factor. PRL is
released by thyrotropin-releasing hormone,
serotonin, and the stress of anesthesia and surgery.
The secretion of PRL is increased by pituitary stalk
section (which interrupts dopaminergic fibers),
serotonin, phenothiazines, alpha-methyldopa, and
menopause.
PRL secretion is primarily regulated by dopamine,
which functions as an inhibitory factor. PRL is
released by thyrotropin-releasing hormone,
serotonin, and the stress of anesthesia and surgery.
The secretion of PRL is increased by pituitary stalk
section (which interrupts dopaminergic fibers),
serotonin, phenothiazines, alpha-methyldopa, and
menopause.
 Normal PRL is 15 to 25 ng/mL.
Normal PRL is 15 to 25 ng/mL.
 Diagnosis. PRL >25 ng/mL is present. Eighty percent
of patients whose PRL exceeds 200 ng/mL
have adenomas even if their presence is not
demonstrated neuroradiologically. When PRL exceeds
2,000 ng/mL, invasion of the cavernous sinus is
likely. This may warrant additional intravenous
access and monitoring with electroencephalogram
(EEG) and evoked potentials.
Diagnosis. PRL >25 ng/mL is present. Eighty percent
of patients whose PRL exceeds 200 ng/mL
have adenomas even if their presence is not
demonstrated neuroradiologically. When PRL exceeds
2,000 ng/mL, invasion of the cavernous sinus is
likely. This may warrant additional intravenous
access and monitoring with electroencephalogram
(EEG) and evoked potentials.
 Amenorrhea. PRL >30 ng/mL is present.
Amenorrhea. PRL >30 ng/mL is present.
 Loss of libido. PRL of >300 ng/mL is present.
Loss of libido. PRL of >300 ng/mL is present.
 Menopause. PRL is increased, and estrogen is
decreased.
Menopause. PRL is increased, and estrogen is
decreased.
 Nonfunctioning pituitary tumors include adenomas,
craniopharyngiomas, meningiomas, and aneurysms.
These tend to be large and involve perisellar
structures.
Nonfunctioning pituitary tumors include adenomas,
craniopharyngiomas, meningiomas, and aneurysms.
These tend to be large and involve perisellar
structures.
III. Endocrine physiology: posterior pituitary
 Antidiuretic hormone (ADH)
Antidiuretic hormone (ADH)
 Produced in the supraoptic and paraventricular
nuclei of the hypothalamus
Produced in the supraoptic and paraventricular
nuclei of the hypothalamus
 Stored in the median eminence of the hypothalamus
Stored in the median eminence of the hypothalamus
 Transported with a carrier protein, neurophysin,
along the hypothalamic hypophyseal tract to the
posterior pituitary
Transported with a carrier protein, neurophysin,
along the hypothalamic hypophyseal tract to the
posterior pituitary
 Released into the systemic circulation after
appropriate stimulus: increased serum osmolality,
pain, opiates, and decreased circulating blood
volume, which causes the greatest ADH release and
concurrent vasoconstriction
Released into the systemic circulation after
appropriate stimulus: increased serum osmolality,
pain, opiates, and decreased circulating blood
volume, which causes the greatest ADH release and
concurrent vasoconstriction
 Secretion inhibited by decreased serum osmolality,
alcohol ingestion, increased blood volume, phenytoin
Secretion inhibited by decreased serum osmolality,
alcohol ingestion, increased blood volume, phenytoin
 ADH attaches to an adenyl cyclase receptor on the
medullary interstitial surface of the renal
collecting duct epithelium. This causes an increase
in cyclic adenosine monophosphate, which increases
the permeability of the collecting ducts to water,
and water is reabsorbed. In the absence of ADH, pure
water is lost.
ADH attaches to an adenyl cyclase receptor on the
medullary interstitial surface of the renal
collecting duct epithelium. This causes an increase
in cyclic adenosine monophosphate, which increases
the permeability of the collecting ducts to water,
and water is reabsorbed. In the absence of ADH, pure
water is lost.
 Diabetes insipidus (DI) can be present
preoperatively, may also occur intraoperatively, and
may be temporary or permanent in the postoperative
period.
Diabetes insipidus (DI) can be present
preoperatively, may also occur intraoperatively, and
may be temporary or permanent in the postoperative
period.
 Signs and symptoms include polyuria (3 to 15 L/day),
polydipsia, serum hyperosmolality (>320 mosmol/mL),
dilute urine (specific gravity 1.001 to 1.005,
osmolality 50 to 150 mosmol/mL), and urine/serum
osmolality <1.
Signs and symptoms include polyuria (3 to 15 L/day),
polydipsia, serum hyperosmolality (>320 mosmol/mL),
dilute urine (specific gravity 1.001 to 1.005,
osmolality 50 to 150 mosmol/mL), and urine/serum
osmolality <1.
 Administration of salt-containing solutions causes
the patient to develop severe hypernatremia and
hyperosmolality. Oral intake is initiated as soon as
possible.
Administration of salt-containing solutions causes
the patient to develop severe hypernatremia and
hyperosmolality. Oral intake is initiated as soon as
possible.
 Administration of glucose-containing solutions
causes the patient to develop hyperglycemia and
osmotic diuresis. Oral intake is initiated as soon
as possible.
Administration of glucose-containing solutions
causes the patient to develop hyperglycemia and
osmotic diuresis. Oral intake is initiated as soon
as possible.
 Total body water deficit calculation in a 70-kg
patient:
Total body water deficit calculation in a 70-kg
patient:
1.
Normal serum sodium, (Na) = 140 mEq/L
2.
Total body water = 60%, of total body weight = 42 L
3.
Normal body sodium = 42 L — 140 mEq/L
= 5,880 mEq Na
4.
Patient's Na = 160 mEq/L
5.
Patient's body water = 5,880 mEq/160 mEq/L
= 36.7 L
6.
Water deficit = 42 L - 36.7 L = 5.3 L
 Drug therapy indicated in patients who cannot drink
the necessary volume, are not taking anything by
mouth, or are anesthetized includes:
Drug therapy indicated in patients who cannot drink
the necessary volume, are not taking anything by
mouth, or are anesthetized includes:
a.
1-Deamino-8-bd-arginine vasopressin (DDAVP), 10 to
40 mcg intranasally. This daily dose may be divided
into 2 or 3 doses beginning with 10 mcg at bedtime
and increased by 2.5 mcg/day up to a total dose of
40 mcg/day. DDAVP, 0.01 to 0.03 ng/kg every 12
hours, may also be given intravenously.
b.
Lysine vasopressin, 5 to 10 units administered
subcutaneously or intramuscularly 2, 3, or 4 times a
day, or 0.5 to 2 microunits/kg/hour administered
intravenously (i.v.).
c.
Other drugs include vasopressin tannate-in-oil,
which is used less commonly. Its effect lasts up to
36 hours after a single dose.
d.
Overdose of drugs leads to an iatrogenic syndrome of
inappropriate antidiuretic hormone (SIADH)
secretion.
IV. Pituitary tumors
 Signs and symptoms
Signs and symptoms
 Headaches are bitemporal or bifrontal.
Headaches are bitemporal or bifrontal.
 Bitemporal hemianopsia is classic but its presence
depends on the relationship of the pituitary tumor
to the optic chiasm and nerves.
Bitemporal hemianopsia is classic but its presence
depends on the relationship of the pituitary tumor
to the optic chiasm and nerves.
 Ophthalmoplegia involving cranial nerves III, IV,
and VI and facial paralysis involving cranial nerve
VII occur. Corneal anesthesia involving cranial
nerve V is related to invasion or compression of the
cavernous sinus.
Ophthalmoplegia involving cranial nerves III, IV,
and VI and facial paralysis involving cranial nerve
VII occur. Corneal anesthesia involving cranial
nerve V is related to invasion or compression of the
cavernous sinus.
 Seizures may be related to the extension of the
tumor into the temporal lobe but are rare.
Seizures may be related to the extension of the
tumor into the temporal lobe but are rare.
 Hypothalamic dysfunction includes abnormal
temperature regulation, thirst, and appetite
changes, all of which are rare.
Hypothalamic dysfunction includes abnormal
temperature regulation, thirst, and appetite
changes, all of which are rare.
 DI may occur.
DI may occur.
 Endocrinopathies. Syndrome of multiple endocrine neoplasia may include parathyroid
dysfunction with hypercalcemia and TSH-, ACTH-, LH-,
and FSH-producing adenomas.
Endocrinopathies. Syndrome of multiple endocrine neoplasia may include parathyroid
dysfunction with hypercalcemia and TSH-, ACTH-, LH-,
and FSH-producing adenomas.
 Diagnosis.
MRI is the gold standard for diagnosing
micro-versus macroadenoma (T1-hypointensity;
T2-hyperintensity) but is poor for visualizing bony
changes and identifying cavernous sinus invasion.
Angiography facilitates hormone sampling from the
petrosal vein.
Diagnosis.
MRI is the gold standard for diagnosing
micro-versus macroadenoma (T1-hypointensity;
T2-hyperintensity) but is poor for visualizing bony
changes and identifying cavernous sinus invasion.
Angiography facilitates hormone sampling from the
petrosal vein.
 Panhypopituitarism is a clinical diagnosis confirmed
by assaying specific hormones.
Panhypopituitarism is a clinical diagnosis confirmed
by assaying specific hormones.
 Most patients who have microadenomas are clinically
normal and do not demonstrate any signs of
panhypopituitarism.
Most patients who have microadenomas are clinically
normal and do not demonstrate any signs of
panhypopituitarism.
 Nonetheless, in some institutions, it is customary
to administer hydrocortisone, 50 to 100 mg i.v.,
before induction of anesthesia and then 10 mg/hour
by infusion until the patient's postoperative course
indicates that the drug is no longer necessary.
Nonetheless, in some institutions, it is customary
to administer hydrocortisone, 50 to 100 mg i.v.,
before induction of anesthesia and then 10 mg/hour
by infusion until the patient's postoperative course
indicates that the drug is no longer necessary.
 Thyroid replacement may be administered orally as
levothyroxine sodium (Synthroid) and very rarely as
an intravenous infusion.
Thyroid replacement may be administered orally as
levothyroxine sodium (Synthroid) and very rarely as
an intravenous infusion.
 DI is treated with DDAVP and appropriate fluids. If panhypopituitarism has been diagnosed
preoperatively, the patient may already be receiving
replacement steroids and DDAVP.
DI is treated with DDAVP and appropriate fluids. If panhypopituitarism has been diagnosed
preoperatively, the patient may already be receiving
replacement steroids and DDAVP.
 Intraoperative management
Intraoperative management
 Monitoring is appropriate for the patient's
physiologic status.
Monitoring is appropriate for the patient's
physiologic status.
1.
Consider either EEG or evoked potential monitoring
if there is marked involvement of the cavernous
sinus or perisellar area.
2.
In some institutions, pituitary operations are
performed in a head-elevated position. Venous air
embolism (VAE) could occur, so end-tidal gas
monitoring is recommended. If, VAE occurs, the head
can be lowered rapidly to treat the air embolism.
The need for central venous access is determined by
the size and location of the tumor and/or by the
patient's medical condition. However, careful
monitoring of fluid intake and output is indicated
in every patient.
3. Visual evoked responses are not monitored in most
institutions and are not indicated for patients who
have microadenomas, but it is desirable when the
tumor reach giant sizes.
 Anesthetic technique is selected to permit early
postoperative assessment of vision, ocular
movements, pupil size, and motor strength.
Anesthetic technique is selected to permit early
postoperative assessment of vision, ocular
movements, pupil size, and motor strength.
 Antibiotic prophylaxis is typically cefazolin, 1 g
i.v. every 3 to 4 hours.
Antibiotic prophylaxis is typically cefazolin, 1 g
i.v. every 3 to 4 hours.
 Topical cocaine 4% and injected lidocaine 1% with
epinephrine; if both are used, severe hypertension
might occur from the unopposed alpha sympathetic
effect.
Topical cocaine 4% and injected lidocaine 1% with
epinephrine; if both are used, severe hypertension
might occur from the unopposed alpha sympathetic
effect.
 Valsalva maneuvers advance the pituitary gland
toward the surgeon to facilitate excision of the
tumor and examination for trans-sellar cerebrospinal
fluid (CSF) leak.
Valsalva maneuvers advance the pituitary gland
toward the surgeon to facilitate excision of the
tumor and examination for trans-sellar cerebrospinal
fluid (CSF) leak.
 Avoid hyperventilation, insertion of nasogastric
tubes, incentive spirometry, and the use of drinking
straws. Hypoventilation (Paco2 42 mm Hg, ICP up to
20 mm Hg) is successful in producing the descent of
the suprasellar portion of the tumor.
Avoid hyperventilation, insertion of nasogastric
tubes, incentive spirometry, and the use of drinking
straws. Hypoventilation (Paco2 42 mm Hg, ICP up to
20 mm Hg) is successful in producing the descent of
the suprasellar portion of the tumor.
 Postoperative management
Postoperative management
 Careful fluid and electrolyte management and
treatment of DI, SIADH, and cerebral salt wasting
are necessary.
Careful fluid and electrolyte management and
treatment of DI, SIADH, and cerebral salt wasting
are necessary.
 Steroid maintenance and tapering. Patients who have
Cushing's disease may have prolonged adrenal
insufficiency and require steroid replacement for
several months.
Steroid maintenance and tapering. Patients who have
Cushing's disease may have prolonged adrenal
insufficiency and require steroid replacement for
several months.
 Acromegalics and patients with Cushing's disease
have excess body water and will diurese
postoperatively.
Acromegalics and patients with Cushing's disease
have excess body water and will diurese
postoperatively.
 Patients who have had previous adrenalectomies
(Nelson's syndrome) require mineralocorticoid
replacement such as fludrocortisone acetate (Florinef),
0.1 to 0.2 mg/day by mouth.
Patients who have had previous adrenalectomies
(Nelson's syndrome) require mineralocorticoid
replacement such as fludrocortisone acetate (Florinef),
0.1 to 0.2 mg/day by mouth.
 Deep vein thrombosis and pulmonary emboli are not
uncommon. Prophylaxis is recommended with heparin,
intermittently inflating antithromboembolism
stockings, and early mobilization.
Deep vein thrombosis and pulmonary emboli are not
uncommon. Prophylaxis is recommended with heparin,
intermittently inflating antithromboembolism
stockings, and early mobilization.
 Lumbar drains may be used postoperatively to treat
CSF leaks.
Lumbar drains may be used postoperatively to treat
CSF leaks.
V. Pituitary apoplexy
 This syndrome is related to the sudden enlargement
of a pituitary tumor because of hemorrhage or
necrosis.
This syndrome is related to the sudden enlargement
of a pituitary tumor because of hemorrhage or
necrosis.
 Symptoms and signs include acute loss of
consciousness, hypertension, meningismus, eye pain,
blindness, ophthalmoplegia, panhypopituitarism. It
is important to differentiate this condition from
subarachnoid hemorrhage from the rupture of an
intracranial aneurysm.
Symptoms and signs include acute loss of
consciousness, hypertension, meningismus, eye pain,
blindness, ophthalmoplegia, panhypopituitarism. It
is important to differentiate this condition from
subarachnoid hemorrhage from the rupture of an
intracranial aneurysm.
 Diagnosis is made on clinical grounds and with
radiologic evidence of a pituitary tumor.
Diagnosis is made on clinical grounds and with
radiologic evidence of a pituitary tumor.
 Treatment is urgent: surgical decompression of the
optic system, systemic steroid replacement, and
other hormone replacement as necessary. Some
recommend bromocriptine therapy in lieu of surgery.
Treatment is urgent: surgical decompression of the
optic system, systemic steroid replacement, and
other hormone replacement as necessary. Some
recommend bromocriptine therapy in lieu of surgery.
VI. Treatment of pituitary tumors
 Radiation therapy is rarely used now because of the
high incidence of panhypopituitarism and the long
lag time for clinical effect, but it is still
showing effective results with recurrence.
Radiation therapy is rarely used now because of the
high incidence of panhypopituitarism and the long
lag time for clinical effect, but it is still
showing effective results with recurrence.
 Medical therapy
Medical therapy
 ACTH (Cushing's disease). Because there is no
effective medical therapy, operative removal is
recommended.
ACTH (Cushing's disease). Because there is no
effective medical therapy, operative removal is
recommended.
 GH (acromegaly). Octreotide, a somatostatin
inhibitor, is an expensive drug ($7,800 annually)
that requires multiple daily subcutaneous
injections. Octreotide reduces headaches and
improves
cardiomyopathy and can be used to treat patients in
whom surgical results have been less than optimal.
However, operative removal is preferred.
GH (acromegaly). Octreotide, a somatostatin
inhibitor, is an expensive drug ($7,800 annually)
that requires multiple daily subcutaneous
injections. Octreotide reduces headaches and
improves
cardiomyopathy and can be used to treat patients in
whom surgical results have been less than optimal.
However, operative removal is preferred.
 PRL. Bromocriptine or similar drugs such as
pergolide are the first-line treatment.
PRL. Bromocriptine or similar drugs such as
pergolide are the first-line treatment.
1.
They reduce tumor size and PRL levels.
2.
They restore fertility. The risks of pregnancy in
the presence of a pituitary tumor include its
enlargement during pregnancy, which may necessitate
operative removal. Perhaps tumor resection should
precede pregnancy.
3.
If patients are intolerant of the drug's side
effects (nausea, dizziness, orthostatic
hypotension), surgery is indicated.
4.
Medical therapy must be continued long term to
indefinitely when surgery is not performed.
 Surgical therapy
Surgical therapy
 Transsphenoidal: Approach to the floor of the sella
is midline, transnasal, transsphenoidal.
Transsphenoidal: Approach to the floor of the sella
is midline, transnasal, transsphenoidal.
1. Advantages include less damage to frontal lobes and
olfactory apparatus, no external scar, direct
visualization of microadenomas, minimal damage to
normal pituitary, lower incidence of temporary and
(rarely) permanent DI, less blood loss, and shorter
hospitalization. Endoscopic transseptal approach to
the sphenoid sinus has been found to be easy, time
saving, and without complications.
2. Disadvantages include the potential for CSF leak and
meningitis, lack of direct visualization of the
optic apparatus, inaccessibility of large tumors,
and blood loss that is more difficult to control.
Bleeding may require packing the cavernous sinus
with resultant compression of the cranial nerves and
carotid artery, which could lead to contralateral
neurologic deficit.
 Transfrontal: bifrontal or unilateral craniotomy
Transfrontal: bifrontal or unilateral craniotomy
1. Advantages include the ability to access suprasellar
tumor extension and visualize optic system and other perisellar structures.
2. Disadvantages are higher morbidity than with
transsphenoidal, increased likelihood of temporary
and permanent DI, possible optic system and vascular
injury, cerebral edema, and longer hospitalization.

|