|
| Head Injury |
Supratentorial Tumors |
Posterior Fossa Surgery|
Intracranial Aneurysms
|Ischemic Cerebrovascular Diseases
|Neuroendocrine Tumors
|Epilepsy-Awake
Craniotomy-Intraoperative MRI
|Spinal Cord Injury and Procedures
|Pediatric Neuroanesthesia
|Neurosurgery in the Pregnant
Patient
|Management of Therapeutic
Interventional Neuroradiology
|Management in Diagnostic
Neuroradiology
|

I. Anesthesia for
supratentorial tumors
 Background.
Approximately 35,000 new brain tumors are diagnosed
per year in the United States. In adults, 85% are
primary (9% of all primary tumors); 60% are primary
and supratentorial (gliomas approximately 35%;
meningiomas approximately 15%; pituitary adenomas
approximately 8%). Approximately 12% of intracranial
tumors are metastases. Their incidence increases
with age, and approximately one-sixth of patients
with cancer develop a brain metastasis which is
symptomatic in most cases and often the controlling
variable for survival.
Background.
Approximately 35,000 new brain tumors are diagnosed
per year in the United States. In adults, 85% are
primary (9% of all primary tumors); 60% are primary
and supratentorial (gliomas approximately 35%;
meningiomas approximately 15%; pituitary adenomas
approximately 8%). Approximately 12% of intracranial
tumors are metastases. Their incidence increases
with age, and approximately one-sixth of patients
with cancer develop a brain metastasis which is
symptomatic in most cases and often the controlling
variable for survival.
 General considerations
General considerations
 Concerns and problems
Concerns and problems
1. Patient symptoms
result from local mass effect and generalized
increased intracranial pressure (ICP) effects.
2. Main surgical concern
is brain exposure without retraction or
mobilization damage.
3. Main anesthetic concern
is the avoidance of secondary brain damage
(Table-1). Therefore, understanding the following is
vital: pathophysiology of ICP and cerebral
perfusion; effects of anesthesia on ICP, cerebral
perfusion, and metabolism; and therapeutic options
for decreasing ICP, brain bulk, and tension
perioperatively.
4. Specific problems
are massive intraoperative hemorrhage, seizures, air
embolism (head-elevated/sitting position or if
venous sinuses are traversed), monitoring brain
function and environment, and rapid versus prolonged
anesthetic emergence. A concurrence of intra- and
extracranial pathologies might also occur (e.g.,
cardiovascular or pulmonary disease; paraneoplastic
phenomena with metastases; chemotherapy/radiotherapy
effects).
 Pathophysiology of rising ICP.
The usual intracranial space-occupying components -
brain tissue, intravascular blood, cerebrospinal
fluid (CSF) are contained in an unyielding skull.
Any volume increase (tumor) must be compensated by
parallel volume reduction of one or more of these
components, mainly CSF or blood (the brain is
largely incompressible). The ability to compensate
for the presence of a mass and maintain homeostasis
depends on the volume of the mass and its rate of
growth (the ICP volume curve shifts to the left for
rapidly expanding masses). Homeostatic mechanisms:
early (limited capacity) intracranial to
extracranial blood shift; late (larger capacity) CSF
displacement (ineffective if CSF flow is
obstructed); exhaustion with rapid ICP rise and
impaired cerebral circulation leading to brain
herniation (end stage of compensation).
Pathophysiology of rising ICP.
The usual intracranial space-occupying components -
brain tissue, intravascular blood, cerebrospinal
fluid (CSF) are contained in an unyielding skull.
Any volume increase (tumor) must be compensated by
parallel volume reduction of one or more of these
components, mainly CSF or blood (the brain is
largely incompressible). The ability to compensate
for the presence of a mass and maintain homeostasis
depends on the volume of the mass and its rate of
growth (the ICP volume curve shifts to the left for
rapidly expanding masses). Homeostatic mechanisms:
early (limited capacity) intracranial to
extracranial blood shift; late (larger capacity) CSF
displacement (ineffective if CSF flow is
obstructed); exhaustion with rapid ICP rise and
impaired cerebral circulation leading to brain
herniation (end stage of compensation).
|
Table-1. Secondary insults
to the already injured brain |
|
Intracranial |
Systemic |
| Increased
intracranial pressure |
Hypercapnia/hypoxemia |
| Epilepsy |
Hypo-/hypertension |
| Vasospasm |
Hypo-/hyperglycemia |
| Herniation: falx,
tentorium, foramen magnum, craniotomy |
Low cardiac output
|
| Midline
shift: tearing of cerebral vessels |
Hypo-osmolality |
| Shivering/pyrexia |
 Intracerebral perfusion and
cerebral blood flow (CBF)
Intracerebral perfusion and
cerebral blood flow (CBF)
 Regulation of CBF is
through gradients in wall pressure of cerebral
arterioles (result of cerebral perfusion pressure
[CPP]) and partial pressure of arterial carbon
dioxide (Paco2) concentration (result of
ventilation) (Figure -1).
Regulation of CBF is
through gradients in wall pressure of cerebral
arterioles (result of cerebral perfusion pressure
[CPP]) and partial pressure of arterial carbon
dioxide (Paco2) concentration (result of
ventilation) (Figure -1).
 Autoregulation of CBF
keeps the CBF constant despite changing CPP via
alterations in cerebral vasomotor tone (i.e.,
cerebrovascular resistance [CVR]). Characteristics
are: dominant to ICP homeostasis; normally
functional for CPP of 50 to 150 mm Hg;
impaired/affected by intracranial (e.g., blood in
CSF, trauma, tumors) and extracranial (e.g., chronic
systemic hypertension) pathologies and anesthetic
drugs. Autoregulation is not immediate in that a
sudden increase in blood pressure gives rise to a
temporary increase in CBF.
Autoregulation of CBF
keeps the CBF constant despite changing CPP via
alterations in cerebral vasomotor tone (i.e.,
cerebrovascular resistance [CVR]). Characteristics
are: dominant to ICP homeostasis; normally
functional for CPP of 50 to 150 mm Hg;
impaired/affected by intracranial (e.g., blood in
CSF, trauma, tumors) and extracranial (e.g., chronic
systemic hypertension) pathologies and anesthetic
drugs. Autoregulation is not immediate in that a
sudden increase in blood pressure gives rise to a
temporary increase in CBF.
 Formulas. CBF =
CPP/CVR, CPP = MAP-ICP. Note that normally, ICP
≈
CVP (central venous pressure).
Formulas. CBF =
CPP/CVR, CPP = MAP-ICP. Note that normally, ICP
≈
CVP (central venous pressure).
 Inadequate perfusion.
Depends on both the reduction of CBF and its
duration when CBF falls under 20 mL/100 g/minute.
Inadequate perfusion is also linked to CPP <50 mm Hg
with intact autoregulation. Action is to restore CPP
and CBF (↑ MAP [mean arterial pressure], ↓ ICP,
↑cardiac output); reduce cerebral metabolic demand
(deepen anesthesia and hypothermia and treat
epilepsy).
Inadequate perfusion.
Depends on both the reduction of CBF and its
duration when CBF falls under 20 mL/100 g/minute.
Inadequate perfusion is also linked to CPP <50 mm Hg
with intact autoregulation. Action is to restore CPP
and CBF (↑ MAP [mean arterial pressure], ↓ ICP,
↑cardiac output); reduce cerebral metabolic demand
(deepen anesthesia and hypothermia and treat
epilepsy).
|
Figure-1.
Pressure-cerebral blood flow relationships. |
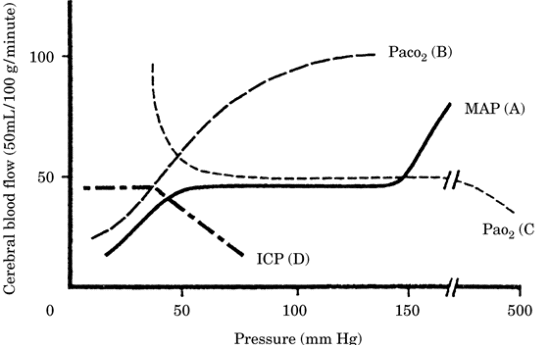 |
|
(A) Cerebral blood flow
(CBF) autoregulation. CBF is maintained at
50 mL/100 g/minute for mean arterial
pressure (MAP)/cerebral perfusion pressure =
50 to 150 mm Hg. (B) Linear relationship
between partial pressure of arterial carbon
dioxide (Paco2) and CBF for Paco2 = 20 to 80
mm Hg. (C) Pao2 and CBF. (D) Intracranial
pressure (ICP) and CBF. |
 Vasodilatory and vasoconstrictive cascades. If
autoregulation is intact:
Vasodilatory and vasoconstrictive cascades. If
autoregulation is intact:
↓ MAP→ cerebral arteriolar vessel dilatation
→↑CBV (cerebral blood
volume) →↑ ICP
→↓ CPP (vicious circle!). Conversely,
↑MAP→↑ CPP
→↓ ICP via cerebral
vasoconstriction (positive circle).
 Paco2. Hypocarbia results in vasoconstriction,
reducing CBF, CBV, and therefore ICP, making
hyperventilation a favorite tool for the acute
control of intracerebral hyperemia and elevated ICP.
However, the relative reduction of CBF is larger
than the reduction of cerebral metabolic rate for
oxygen consumption (CMRo2), inducing a risk of
cerebral ischemia.
Paco2. Hypocarbia results in vasoconstriction,
reducing CBF, CBV, and therefore ICP, making
hyperventilation a favorite tool for the acute
control of intracerebral hyperemia and elevated ICP.
However, the relative reduction of CBF is larger
than the reduction of cerebral metabolic rate for
oxygen consumption (CMRo2), inducing a risk of
cerebral ischemia.
 Anesthesia and intracranial pressure, perfusion, and
metabolism. Anesthesia affects the intracranial
environment through drug and nondrug effects, all
sensitive to the intra- and extracranial state
(e.g., cerebral compliance, intracranial pathology, volemic state).
Anesthesia and intracranial pressure, perfusion, and
metabolism. Anesthesia affects the intracranial
environment through drug and nondrug effects, all
sensitive to the intra- and extracranial state
(e.g., cerebral compliance, intracranial pathology, volemic state).
 Intravenous anesthetics (barbiturates, propofol,
etomidate) reduce CMRo2 dose dependently by
depressing electrical and neurotransmitter synthesis
(not basal metabolic) activity of the neurons with a
ceiling effect at electroencephalographic (EEG)
burst suppression. They are cerebral
vasoconstrictors →↓ CBF, CBV, and ICP. Cerebral flow-metabolism
coupling, autoregulation, and Paco2 vessel
reactivity remain intact. In contrast to volatile
anesthetics, propofol suppresses the cerebrostimulatory effects of nitrous oxide.
Intravenous anesthetics (barbiturates, propofol,
etomidate) reduce CMRo2 dose dependently by
depressing electrical and neurotransmitter synthesis
(not basal metabolic) activity of the neurons with a
ceiling effect at electroencephalographic (EEG)
burst suppression. They are cerebral
vasoconstrictors →↓ CBF, CBV, and ICP. Cerebral flow-metabolism
coupling, autoregulation, and Paco2 vessel
reactivity remain intact. In contrast to volatile
anesthetics, propofol suppresses the cerebrostimulatory effects of nitrous oxide.
 Volatile anesthetics (e.g., isoflurane, sevoflurane,
desflurane) decrease CMRo2. They are all cerebral
vasodilators (desflurane > isoflurane >
sevoflurane). For <1 to 1.5 minimum alveolar
concentration (MAC) and in the normal brain
(flow/metabolism coupling intact), CBF decreases
compared to the awake state and autoregulation is
maintained. Above 1 to 1.5 MAC, there is a
dose-related increase in CBF with impaired
autoregulation (Figures -2 and -3). Maintained
Paco2 reactivity allows hypo-capnic control of this
type of vasodilatation. A situation to avoid is
brain pathology + high volatile MAC
→
impaired/abolished carbon dioxide (CO2) reactivity.
Volatile anesthetics (e.g., isoflurane, sevoflurane,
desflurane) decrease CMRo2. They are all cerebral
vasodilators (desflurane > isoflurane >
sevoflurane). For <1 to 1.5 minimum alveolar
concentration (MAC) and in the normal brain
(flow/metabolism coupling intact), CBF decreases
compared to the awake state and autoregulation is
maintained. Above 1 to 1.5 MAC, there is a
dose-related increase in CBF with impaired
autoregulation (Figures -2 and -3). Maintained
Paco2 reactivity allows hypo-capnic control of this
type of vasodilatation. A situation to avoid is
brain pathology + high volatile MAC
→
impaired/abolished carbon dioxide (CO2) reactivity.
 Nitrous oxide (N2O) is cerebrostimulatory
→↑
CMRo2, CBF, and sometimes ICP, particularly with
volatile anesthesia. For the
normal brain, this cerebral vasodilatation can be
controlled by hypocapnia or intravenous anesthetics
(volatiles - no attenuating effect). CMRo2 and CBF
are higher for 1 MAC anesthesia with nitrous
oxide-volatile versus volatile only.
Nitrous oxide (N2O) is cerebrostimulatory
→↑
CMRo2, CBF, and sometimes ICP, particularly with
volatile anesthesia. For the
normal brain, this cerebral vasodilatation can be
controlled by hypocapnia or intravenous anesthetics
(volatiles - no attenuating effect). CMRo2 and CBF
are higher for 1 MAC anesthesia with nitrous
oxide-volatile versus volatile only.
|
Figure-2. Cerebral blood flow (CBF)-cerebral
metabolic rate for oxygen consumption (CMRo2)
coupling during increasing dose of an intravenous
anesthetic. A normal CMRo2 of 4 mL/100
g/minute is coupled to a CBF of 50 mL/100
g/minute. EEG, electroencephalogram. |
|
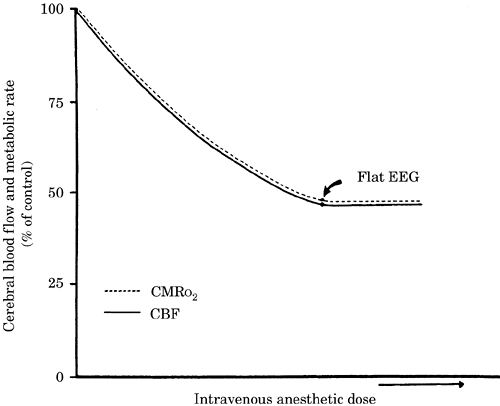 |
|
Figure-3. Cerebral blood flow (CBF)-cerebral
metabolic rate for oxygen consumption
(CMRo2)-cerebral blood volume (CBV) during
increasing doses of an intravenous (propofol) and
volatile anesthetics. Changes are noted as a
percentage value from the awake state. Despite
similar changes in CMRo2, changes in CBF and CBV are
markedly different among intravenous and volatile
agents and among sevoflurane, isoflurane, and
desflurane above 1.5 minimum alveolar concentration
(MAC). EC50, median effective concentration. |
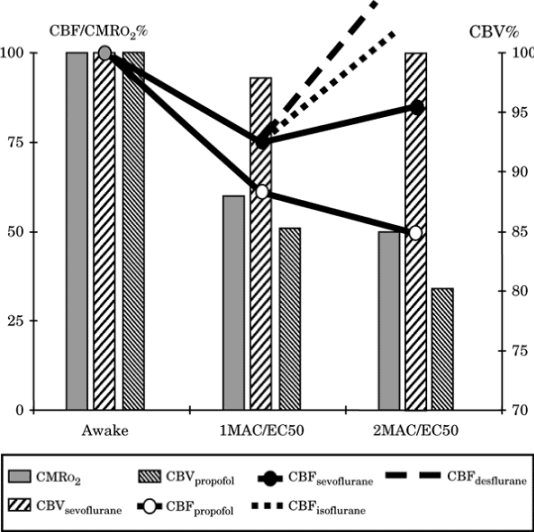 |
 Opioids have been associated with short-term
↑ ICP
(large doses). However, opioids are only modest
direct cerebral vasodilators; therefore, reflex
cerebral vasodilatation after ↓ MAP/CPP probably
causes the transient ↑ ICP. Opioids modestly
↓
CMRo2 without affecting flow-metabolism coupling,
autoregulation, or vessel CO2 sensitivity.
Remifentanil is particularly suitable for rapid
emergence.
Opioids have been associated with short-term
↑ ICP
(large doses). However, opioids are only modest
direct cerebral vasodilators; therefore, reflex
cerebral vasodilatation after ↓ MAP/CPP probably
causes the transient ↑ ICP. Opioids modestly
↓
CMRo2 without affecting flow-metabolism coupling,
autoregulation, or vessel CO2 sensitivity.
Remifentanil is particularly suitable for rapid
emergence.
|
Table -2. Intracranial hypertension and brain
bulging: prevention and treatment |
|
Prevention |
Treatment |
| Preoperative: adequate anxiolysis and analgesia
|
Cerebrospinal fluid drainage (lumbar catheter or
ventricle) |
| Preinduction: hyperventilate on demand, head-up
position, head straight, no jugular vein compression
|
Osmotic diuretics |
| Avoid overhydration |
Hyperventilation
|
| Osmotic diuretics (mannitol, hypertonic saline);
steroids for tumor |
Augment depth of anesthesia using intravenous
anesthetics (propofol, thiopental, etomidate) |
| Loop diuretics (furosemide)
|
Muscle relaxation |
| Optimize hemodynamics: MAP, central venous pressure,
pulmonary capillary wedge pressure, heart rate; use
beta-blockers, clonidine, or lidocaine if necessary |
Improve cerebral venous drainage: head up, no
positive end-expiratory pressure, reduce inspiratory
time |
| Ventilation: Pao2 >100; Paco2 ~ 35 mm Hg, low
intrathoracic pressure |
Mild controlled hypertension if cerebral
autoregulation intact (MAP ~ 100 mm Hg) |
| Use of intravenous anesthetics for induction and
maintenance |
|
|
MAP, mean arterial pressure.
|
 Neuromuscular blocking agents (NMB). The effect of
succinylcholine on ICP is controversial, but fasciculations may increase ICP. Succinylcholine may
be used for difficult intubation or rapid sequence
induction in patients with brain injuries. Other NMB
have no effect on ICP.
Neuromuscular blocking agents (NMB). The effect of
succinylcholine on ICP is controversial, but fasciculations may increase ICP. Succinylcholine may
be used for difficult intubation or rapid sequence
induction in patients with brain injuries. Other NMB
have no effect on ICP.
 Other drugs. Avoid vasodilating antihypertensive
agents (nitroglycerin, nitroprusside, hydralazine)
↑ cerebral vasodilatation. Take into account
pharmacologic interactions, particularly with
antiepileptic agents.
Other drugs. Avoid vasodilating antihypertensive
agents (nitroglycerin, nitroprusside, hydralazine)
↑ cerebral vasodilatation. Take into account
pharmacologic interactions, particularly with
antiepileptic agents.
 Reducing ICP, brain bulk, and tension (Table-2).
The effectiveness of these techniques depends on
intact intracerebral homeostatic mechanisms and/or
structures.
Reducing ICP, brain bulk, and tension (Table-2).
The effectiveness of these techniques depends on
intact intracerebral homeostatic mechanisms and/or
structures.
 Intravenous anesthetics
→↓ CMRo2, CBF
→↓ CBV, ICP
→↓ brain bulk. Cerebral
vasoconstriction depends on intact CMRo2-CBF
coupling (Figure-2) and is dose related up to
neuronal electrical silence (EEG burst suppression).
Like autoregulation,
CMRo2-CBF coupling is impaired by brain contusion
and other intracerebral pathologies.
Intravenous anesthetics
→↓ CMRo2, CBF
→↓ CBV, ICP
→↓ brain bulk. Cerebral
vasoconstriction depends on intact CMRo2-CBF
coupling (Figure-2) and is dose related up to
neuronal electrical silence (EEG burst suppression).
Like autoregulation,
CMRo2-CBF coupling is impaired by brain contusion
and other intracerebral pathologies.
 Hyperventilation - hypocarbia
- cerebral
vasoconstriction (acute effect lasting for a maximum
of 24 hours). For intact autoregulation, CBF is
linearly related to Paco2 from 20 to 70 mm Hg (3% to
4% change/mm Hg Paco2). Factors impairing CO2
reactivity are head injury, other intracerebral
pathology, high-inspired volatile anesthetic
concentrations, N2O (especially with already dilated
vessels). Typical target: Paco2 of 30 to 35 mm Hg;
based on arterial blood gas analysis rather than
end-tidal CO2 (ETco2: possibility of large
arterio-alveolar CO2 gradients in neurosurgical
patients). Side effects of hyperventilation include
linear reduction in coronary artery flow and cardiac
venous return as well as hypokalemia.
Hyperventilation - hypocarbia
- cerebral
vasoconstriction (acute effect lasting for a maximum
of 24 hours). For intact autoregulation, CBF is
linearly related to Paco2 from 20 to 70 mm Hg (3% to
4% change/mm Hg Paco2). Factors impairing CO2
reactivity are head injury, other intracerebral
pathology, high-inspired volatile anesthetic
concentrations, N2O (especially with already dilated
vessels). Typical target: Paco2 of 30 to 35 mm Hg;
based on arterial blood gas analysis rather than
end-tidal CO2 (ETco2: possibility of large
arterio-alveolar CO2 gradients in neurosurgical
patients). Side effects of hyperventilation include
linear reduction in coronary artery flow and cardiac
venous return as well as hypokalemia.
 Diuretics. Osmotic diuretics (e.g., mannitol,
hypertonic saline) acutely
↑ blood osmolality
→↓ brain water content
(mainly healthy brain tissue with intact blood-brain
barrier) →↓
brain bulk, ICP, ↑ compliance. Also: better blood rheology (↓ endothelial edema;
↓ erythrocyte edema
→↓ erythrocyte deformability). Typical
regimen: mannitol, 0.5 to 1 g/kg intravenously
(i.v.), (split between rapid precraniotomy dose and
slower infusion until brain dissection is complete).
ICP effect: prompt, lasts for 2 to 3 hours, removes
approximately 90 mL brain water at peak effect.
Problems: hypernatremia, acute hypervolemia.
Compensate urinary losses due to mannitol with
isotonic saline.
Diuretics. Osmotic diuretics (e.g., mannitol,
hypertonic saline) acutely
↑ blood osmolality
→↓ brain water content
(mainly healthy brain tissue with intact blood-brain
barrier) →↓
brain bulk, ICP, ↑ compliance. Also: better blood rheology (↓ endothelial edema;
↓ erythrocyte edema
→↓ erythrocyte deformability). Typical
regimen: mannitol, 0.5 to 1 g/kg intravenously
(i.v.), (split between rapid precraniotomy dose and
slower infusion until brain dissection is complete).
ICP effect: prompt, lasts for 2 to 3 hours, removes
approximately 90 mL brain water at peak effect.
Problems: hypernatremia, acute hypervolemia.
Compensate urinary losses due to mannitol with
isotonic saline.
 CSF drainage. Can be accomplished either by direct
puncture of the lateral ventricle by the surgeon or
lumbar spinal catheter by the anesthesiologist
preoperatively; this is effective only without
caudal block to CSF outflow. Acute brain herniation
might occur; therefore, lumbar CSF drainage should
be used cautiously and only when the dura is open
and the patient is at least mildly hyperventilated.
Draining 10 to 20 mL CSF effectively reduces brain
tension; up to 50 mL can be drained if necessary.
CSF drainage. Can be accomplished either by direct
puncture of the lateral ventricle by the surgeon or
lumbar spinal catheter by the anesthesiologist
preoperatively; this is effective only without
caudal block to CSF outflow. Acute brain herniation
might occur; therefore, lumbar CSF drainage should
be used cautiously and only when the dura is open
and the patient is at least mildly hyperventilated.
Draining 10 to 20 mL CSF effectively reduces brain
tension; up to 50 mL can be drained if necessary.
 Use the vasoconstrictive cascade. Mild
↑ MAP
→↑ CPP →↓ CBV.
Use the vasoconstrictive cascade. Mild
↑ MAP
→↑ CPP →↓ CBV.
 Avoid other factors causing cerebral vasodilatation: hypovolemia,
hypoxia, patient positioning (head-down, extreme
turning of the neck →↓
cerebral venous drainage, rotation of the head on
one side and jugular venous
thrombosis on the other side → major brain
swelling), volatile anesthetics >1 to 1.5 MAC.
Avoid other factors causing cerebral vasodilatation: hypovolemia,
hypoxia, patient positioning (head-down, extreme
turning of the neck →↓
cerebral venous drainage, rotation of the head on
one side and jugular venous
thrombosis on the other side → major brain
swelling), volatile anesthetics >1 to 1.5 MAC.
|
Table -3. Preoperative neurologic evaluation |
| History |
| Seizures, level of consciousness
|
|
↑ICP: headache, nausea, vomiting, blurred vision |
| Focal neurology: hemiparesis, sensory deficits, etc. |
| Hydration: duration of bed rest, fluid intake,
diuretics, syndrome of inappropriate secretion of
antidiuretic hormone |
| Medication: steroids, antiepileptic drugs
|
| Associated illnesses, trauma |
| Physical Examination |
| Mental status, level of consciousness
|
| Papilledema (↑CP), Cushing response
(hypertension, bradycardia) |
| Pupil size, speech deficit, Glasgow Coma Scale
score, focal signs |
| Investigations (Computed Tomographic/Magnetic
Resonance Imaging Scans) |
| Size and location of the tumor: e.g., silent or
eloquent area?, near major vessel? |
| Intracranial mass effects: midline shift, ā†“
ventricle size, temporal lobe herniation,
cerebrospinal fluid space surrounding the brain
stem, edema, hydrocephalus |
| ICP, intracranial pressure.
|
 General anesthetic management
General anesthetic management
 Preoperative assessment. Anesthetic strategy is
based on the patient's neurologic and general state
and the planned surgery; both should be discussed
with the neurosurgeon.
Preoperative assessment. Anesthetic strategy is
based on the patient's neurologic and general state
and the planned surgery; both should be discussed
with the neurosurgeon.
 Neurologic state of patient. Assess (Table-3): ICP
increases and intracranial compliance (computed
tomographic [CT] scan or magnetic resonance imaging
[MRI]); size of ICP/CBF homeostatic reserve (margin
before brain ischemia/neurologic impairment);
autoregulation impairment (diffuse brain pathology,
coma); presence of neurologic damage
(permanent/reversible); present drug therapy
(especially antiepileptic drugs and their side
effects); neurodiagnostic studies.
Neurologic state of patient. Assess (Table-3): ICP
increases and intracranial compliance (computed
tomographic [CT] scan or magnetic resonance imaging
[MRI]); size of ICP/CBF homeostatic reserve (margin
before brain ischemia/neurologic impairment);
autoregulation impairment (diffuse brain pathology,
coma); presence of neurologic damage
(permanent/reversible); present drug therapy
(especially antiepileptic drugs and their side
effects); neurodiagnostic studies.
 General state of patient. Cardiovascular system:
brain perfusion/oxygenation depends on it; acute
intracranial pathologies affect cardiac and lung
function (worst situation: neurogenic pulmonary
edema); supratentorial surgery (meningioma,
metastasis) may result in significant bleeding (hypovolemia,
hypotension →↓ CPP/CBF and
↑ ICP).
Respiratory system: hyperventilation to
↑ ICP, CBF, CBV, and brain tension depend on it;
40% of brain metastases are from lung (primary
tumor, its chemotherapy/radiotherapy). The
head-up/sitting position affects the cardiac and
respiratory systems. Other systems: paraneoplastic
or chemotherapy/radiotherapy-associated syndromes
(hematology, coagulation); renal system, diuretics,
and decreased fluid intake; altered endocrine system
(intracranial processes; pituitary adenoma or its
therapy; steroids); gastrointestinal tract (steroids
and mucosa; motility effects of ↑ ICP).
Coagulation profile must be normal: stop aspirin at
least 7 days and clopidogrel 10 days before surgery.
General state of patient. Cardiovascular system:
brain perfusion/oxygenation depends on it; acute
intracranial pathologies affect cardiac and lung
function (worst situation: neurogenic pulmonary
edema); supratentorial surgery (meningioma,
metastasis) may result in significant bleeding (hypovolemia,
hypotension →↓ CPP/CBF and
↑ ICP).
Respiratory system: hyperventilation to
↑ ICP, CBF, CBV, and brain tension depend on it;
40% of brain metastases are from lung (primary
tumor, its chemotherapy/radiotherapy). The
head-up/sitting position affects the cardiac and
respiratory systems. Other systems: paraneoplastic
or chemotherapy/radiotherapy-associated syndromes
(hematology, coagulation); renal system, diuretics,
and decreased fluid intake; altered endocrine system
(intracranial processes; pituitary adenoma or its
therapy; steroids); gastrointestinal tract (steroids
and mucosa; motility effects of ↑ ICP).
Coagulation profile must be normal: stop aspirin at
least 7 days and clopidogrel 10 days before surgery.
 Biology. Coagulation, hemoglobin, platelet count,
potassium, sodium.
Biology. Coagulation, hemoglobin, platelet count,
potassium, sodium.
 Planned operative intervention. Clarify surgical
approach (tumor size/position, proximal structures
and likelihood of vascular involvement, radical
excision), resultant patient positioning (supine,
prone, sitting, lateral), and tumor type.
Planned operative intervention. Clarify surgical
approach (tumor size/position, proximal structures
and likelihood of vascular involvement, radical
excision), resultant patient positioning (supine,
prone, sitting, lateral), and tumor type.
(1) Meningiomas. The combination of large size,
difficult location, and radical excision (total
resection is virtually curative) makes for long,
technically demanding operations, often with
significant bleeding (surrounding structures, meningioma vascularity). Anesthetic priority:
maximal brain tension reduction to facilitate
surgical access; compensate blood losses with
isotonic saline or colloids (hematocrit >28%).
(2) Gliomas. Often simple debulking with easy
surgical access and little risk of bleeding. Risk of
postoperative intracranial hypertension due to
edema.
(3) Others. Third ventricle colloid cysts, which may
result in obstructive hydrocephalus and therefore
↑ ICP at induction. Colloid cysts, basal cistern epidermoids, and transcranially resected pituitary
tumors need maximal brain relaxation for exposure at
skull base.
(4) Pituitary adenoma by transsphenoidal resection.
Essentially an extracranial operation in a head-up
position.
 Determination of anesthetic strategy. Points to be
addressed:
Determination of anesthetic strategy. Points to be
addressed:
(1) Vascular access. Consider the risk of bleeding
or venous air embolism, hemodynamic and metabolic
monitoring, and
infusion needs for vasoactive and other substances.
(2) Fluid therapy. Target normovolemia/normotension;
avoid hyposmolar (Ringer's lactate) and
glucose-containing solutions (hyperglycemia
→↑
ischemic brain injury).
(3) Anesthetic regimen. Simple¯
procedures (low risk of ICP problems or ischemia,
little need for brain relaxation): volatile-based
technique okay (<1.5 MAC). High-risk procedures (anticipated
ICP problems, significant risk of intraoperative
cerebral ischemia, need for deep brain relaxation):
use total intravenous anesthesia with propofol.
(4) Extracranial monitoring such as cardiovascular
or renal, venous air embolism.
(5) Intracranial monitoring. General or local
environment versus specific functions: metabolic
(jugular venous bulb oxygen saturation [Sjo2], brain
tissue oxygen partial pressure [btPo2)],
neurophysiologic (EEG/evoked potential), functional
(transcranial Doppler).
 Preoperative preparation
Preoperative preparation
 Premedication. Risk assessment: sedation
→ hypercapnia, hypoxemia,
upper airway obstruction →↑
ICP; stress →↑ CPP/CBF/CMRo2,
↑ICP
and the development of vasogenic edema with impaired
autoregulation. Best: titrated intravenous
analgesia/sedation (e.g., midazolam, 0.5 to 2 mg, ±
fentanyl, 25 to 100 mcg, or sufentanil, 5 to 20 mcg)
under direct anesthesiologic supervision for
vascular access placement, and so on. Patients
without signs of ↑ICP can benefit from oral
premedication with a small benzodiazepine dose
(e.g., 5 mg midazolam). Continue steroids
(supplement with pituitary axis suppression) and
other regular medication (anticonvulsants, antihypertensives, other cardiac drugs). Consider
starting anticonvulsant therapy if not already
initiated (e.g., loading dose of phenytoin, 15
mg/kg, or fosphenytoin, 20 mg/kg, over 30 minutes)
and H2 blockers (for ↓ gastric emptying,
↑acid
secretion with steroids, ↑ICP).
Premedication. Risk assessment: sedation
→ hypercapnia, hypoxemia,
upper airway obstruction →↑
ICP; stress →↑ CPP/CBF/CMRo2,
↑ICP
and the development of vasogenic edema with impaired
autoregulation. Best: titrated intravenous
analgesia/sedation (e.g., midazolam, 0.5 to 2 mg, ±
fentanyl, 25 to 100 mcg, or sufentanil, 5 to 20 mcg)
under direct anesthesiologic supervision for
vascular access placement, and so on. Patients
without signs of ↑ICP can benefit from oral
premedication with a small benzodiazepine dose
(e.g., 5 mg midazolam). Continue steroids
(supplement with pituitary axis suppression) and
other regular medication (anticonvulsants, antihypertensives, other cardiac drugs). Consider
starting anticonvulsant therapy if not already
initiated (e.g., loading dose of phenytoin, 15
mg/kg, or fosphenytoin, 20 mg/kg, over 30 minutes)
and H2 blockers (for ↓ gastric emptying,
↑acid
secretion with steroids, ↑ICP).
 Vascular access. Two large-bore peripheral
intravenous catheters are typical for full
craniotomy.
Vascular access. Two large-bore peripheral
intravenous catheters are typical for full
craniotomy.
(1) Central venous access. Recommended for
significant risk of venous air embolism (radiographically
control catheter tip
position at transition of vena cava/right atrium) or
bleeding, long-lasting procedures (>6 hours), major
cardiovascular compromise (if severe, consider
pulmonary artery catheter or transesophageal
echocardiography), and continuous infusion of
vasoactive drugs. Jugular cannulation technique
(conventional or retrograde) must be meticulous,
impairment of cerebral venous drainage must be
avoided (hematoma, head-down position
→↑ICP!).
(2) Arterial cannulation. Obligatory for full
craniotomy due to the need for close monitoring and
control of CPP (obtain by transducing arterial
pressure at mid-ear/circle of Willis level, CPP =
MAP - ICP); frequent determination of arterial Paco2
(hyperventilation) and plasma glucose, potassium,
and so on, values. Note that ETco2 monitoring is no
substitute for Paco2 measurement (correlates poorly,
especially with ventilation-perfusion mismatch).
(3) Jugular venous bulb monitoring (JVBM). Permits
monitoring (intermittent or continuous with
fiberoptic oximetry) of cerebral oxygen extraction
(Sao2-Sjvo2), allowing conclusions about the
adequacy of global cerebral perfusion (assuming
CMRo2 is constant). But frequently difficult to
interpret during surgery due to the rotation of the
head. Technique: retrograde cannulation of jugular
vein; catheter tip should be radiographically
verified to be in the jugular venous bulb.
 Monitoring
Monitoring
(1) Cardiovascular. Electrocardiographic (myocardial
ischemia, arrhythmias); arterial and CVP, pulse oximetry. Others: ETco2
(trend monitor for Paco2, detection of venous air
embolism); temperature via esophageal thermistor
(modest, passive hypothermia, [approximately 35°C]
might confer significant neuronal protection during
focal ischemia at small systemic cardiorespiratory
risk); urinary catheter.
(2) Air embolism. Sensitively detected by precordial
Doppler, end-tidal nitrogen or CO2 (alternative: transesophageal echocardiography).
(3) Neuromuscular block. Do not monitor on
hemiplegic extremities (↑ acetylcholine receptor
density of lower motor neuron units innervated by
dysfunctional or nonfunctional upper motor neurons
→ resistance to nondepolarizing myorelaxants
→
effective overdose for normal neuromuscular units). Contralateral hemiparesis to a supratentorial tumor
is not associated with hyperkalemia as in paraplegic
or patients with burns; succinylcholine is therefore
not contraindicated.
(4) Blood chemistry.
Monitor glucose regularly; hyperglycemia
→↑ neuronal damage during
ischemia. During general anesthesia, steroids
→↑ blood glucose levels; brain retraction
→
focal cerebral ischemia. Others: K, hematocrit,
coagulation.
(5) Intracranial environment, cerebral function. JVBM; EEG monitoring
(information on CMRo2, cerebral ischemia, depth
of anesthesia). Others: evoked potentials
(intactness of specific central nervous system [CNS]
pathways); btPo2 (information on adequate oxygen
supply to brain areas at risk of ischemia).
(6) ICP monitoring. Currently rare for elective
neurosurgery due to improvements in perioperative
ICP control but still has an important role in neurotraumatology.
 Induction of anesthesia
Induction of anesthesia
 Goals. Ventilatory control (early mild
hyperventilation; avoid hypercapnia, hypoxemia);
sympathetic/blood pressure control (avoid CNS
arousal: adequate antinociception, anesthesia);
optimal position on ICP-volume curve (avoid venous
outflow obstruction).
Goals. Ventilatory control (early mild
hyperventilation; avoid hypercapnia, hypoxemia);
sympathetic/blood pressure control (avoid CNS
arousal: adequate antinociception, anesthesia);
optimal position on ICP-volume curve (avoid venous
outflow obstruction).
 Typical induction scheme. Detailed in Table-4.
Typical induction scheme. Detailed in Table-4.
 Myorelaxants. Modern nondepolarizing drugs have
minimal effects on intracerebral hemodynamics.
Interaction (↑doses by 50% to 60%) between pancuronium /vecuronium/ rocuronium/ cisatracurium and
chronic (>7 days) phenytoin/carbamazepine treatment
can occur due to increased metabolism and resistance
to myorelaxants; no neuromuscular transmission
monitoring on hemiplegic extremities. Note that because neurosurgical patients are
susceptible to myorelaxant hangover (difficult to
detect by
manual relaxometry), avoid long-acting myorelaxants
(e.g., pancuronium); use middle- to short-acting
drugs (e.g., vecuronium, cisatracurium, mivacurium,
rocuronium).
Myorelaxants. Modern nondepolarizing drugs have
minimal effects on intracerebral hemodynamics.
Interaction (↑doses by 50% to 60%) between pancuronium /vecuronium/ rocuronium/ cisatracurium and
chronic (>7 days) phenytoin/carbamazepine treatment
can occur due to increased metabolism and resistance
to myorelaxants; no neuromuscular transmission
monitoring on hemiplegic extremities. Note that because neurosurgical patients are
susceptible to myorelaxant hangover (difficult to
detect by
manual relaxometry), avoid long-acting myorelaxants
(e.g., pancuronium); use middle- to short-acting
drugs (e.g., vecuronium, cisatracurium, mivacurium,
rocuronium).
|
Table -4. Suggested anesthesia induction and
maintenance scheme |
| Induction |
| Adequate preoperative anxiolysis in the anesthetic
room |
| Electrocardiogram, capnometer, pulse oximeter,
noninvasive blood pressure |
| Venous, arterial lines: insert under LA |
| Furosemide 1 mg/kg |
| Preoxygenation, then fentanyl, 1-2 mcg/kg, (or
alfentanil, sufentanil, remifentanil) |
| Propofol, 1.25-2.5 mg/kg, or thiopental, 3-6
mg/kg, then nondepolarizing myorelaxant |
| Control ventilation (Paco2 ~ 35 mm Hg) |
| Intubation |
| Maintenance |
| Propofol, 50-150 mcg/kg/min, or sevoflurane,
0.5%-1.5%, or desflurane, 3%-6% |
| Maintain analgesia: fentanyl, 1-2 mcg/kg/h, (or
alfentanil, sufentanil, remifentanil) |
| LA, fentanyl 2 mcg/kg (skull-pin head holder
placement, skin incision) |
| Position: head-up, jugular veins free |
| Mannitol, 0.5-0.75 g/kg, insert lumbar drain |
| Ensure adequate volemia (NaCl 0.9% or hydroxyethyl
starch 6%-not Ringer'slactate) |
| LA, local anesthesia.
|
 Patient positioning. Pin holder application is a
maximal nociceptive stimulus. Block by deeper
analgesia (fentanyl bolus, 1 to 3 mcg/kg, sufentanil
bolus, 0.2 to 0.3 mcg/kg, alfentanil, 10 to 20
mcg/kg, remifentanil, 0.25 to 1 mcg/kg) or
anesthesia (e.g., propofol bolus, 0.5 mg/kg) and/or
local anesthetic infiltration of the pin site.
Alternative: antihypertensives (esmolol, 0.5 mg/kg,
labetalol, 0.075 to 0.15 mg/kg). Remember that pin
insertion can introduce venous air embolism! Avoid
extreme positions; pad and/or fix regions
susceptible to pressure, abrasion, or movement
injury. Fix the endotracheal tube securely to avoid
accidental extubation and abrasions with movement,
and tape the eyes occlusively to avoid corneal
damage. A mild head-up position helps venous
drainage; mild knee flexion decreases back strain.
Avoid severe lateral extension/flexion of head on
neck (maintain more than two fingers' space between
chin and
nearest bone). Extreme flexion of the head may
induce quadriparesis or massive swelling of the face
and tongue making rapid extubation impossible. If
the head is turned laterally, elevate contralateral
shoulder (with a wedge or roll) to prevent brachial
plexus stretch injury. Lateral/sitting/prone
position: specific precautions. Verify cautiously
all potential pressure points (eyes),
peripheral arterial pulses, nerve compression, and
ventilation.
Patient positioning. Pin holder application is a
maximal nociceptive stimulus. Block by deeper
analgesia (fentanyl bolus, 1 to 3 mcg/kg, sufentanil
bolus, 0.2 to 0.3 mcg/kg, alfentanil, 10 to 20
mcg/kg, remifentanil, 0.25 to 1 mcg/kg) or
anesthesia (e.g., propofol bolus, 0.5 mg/kg) and/or
local anesthetic infiltration of the pin site.
Alternative: antihypertensives (esmolol, 0.5 mg/kg,
labetalol, 0.075 to 0.15 mg/kg). Remember that pin
insertion can introduce venous air embolism! Avoid
extreme positions; pad and/or fix regions
susceptible to pressure, abrasion, or movement
injury. Fix the endotracheal tube securely to avoid
accidental extubation and abrasions with movement,
and tape the eyes occlusively to avoid corneal
damage. A mild head-up position helps venous
drainage; mild knee flexion decreases back strain.
Avoid severe lateral extension/flexion of head on
neck (maintain more than two fingers' space between
chin and
nearest bone). Extreme flexion of the head may
induce quadriparesis or massive swelling of the face
and tongue making rapid extubation impossible. If
the head is turned laterally, elevate contralateral
shoulder (with a wedge or roll) to prevent brachial
plexus stretch injury. Lateral/sitting/prone
position: specific precautions. Verify cautiously
all potential pressure points (eyes),
peripheral arterial pulses, nerve compression, and
ventilation.
 Maintenance of anesthesia (Table-4)
Maintenance of anesthesia (Table-4)
 Goals
Goals
(1) Controlling brain tension through control of
CMRo2 and CBF. Preventing CNS arousal (depth of
anesthesia, antinociception); treating consequences
of CNS arousal (sympatholysis, antihypertensives);
the chemical brain retractor concept¯ (Table-5).
(2) Neuroprotection. Maintenance of an optimal
intracranial environment (adequate CPP, Paco2, Sao2:
matching cerebral substrate demand and supply);
specific neuroprotection is controversial and should
not induce adverse effects or delayed recovery.
 Choice of technique. Controversy: intravenous or
volatile anesthesia for neurosurgery? No study to
date has shown significant outcome differences for
intravenous versus volatile-based neuroanesthesia.
But operative conditions are worse with volatile
anesthetic inspired concentration (Fi) >1.5 MAC.
Choice of technique. Controversy: intravenous or
volatile anesthesia for neurosurgery? No study to
date has shown significant outcome differences for
intravenous versus volatile-based neuroanesthesia.
But operative conditions are worse with volatile
anesthetic inspired concentration (Fi) >1.5 MAC.
(1) Volatiles. Con: CBF-CMRo2 uncoupling;
↑CBF/ICP/brain bulk. Pro:
easy, extensive, successful use; control;
predictability (early awakening). Recommendation:
use for simple cases (no
ischemia, ICP, or brain bulk problems);
early moderate hyperventilation; Fi < 1.5 MAC; avoid
combination with N2O (↑cerebrostimulation).
|
Table -5. The chemical brain retractor concept
|
| Mild hyperosmolalitya
|
| Mild hyperventilation |
| combined with: |
| Adequate head-up positioning |
| Lumbar cerebrospinal fluid drainage
|
| Intravenous anesthetic agent (propofol)
|
| Mild controlled hypertensionb |
| Avoidance of brain retractors
|
| Venous drainage: jugular veins free |
aBefore bone flap removal, give mannitol, 0.5-0.75
g/kg, or 7.5% NaCl, 3-5 mL/kg (NaCl 0.9% = 304
mOsm/kg).
bMean arterial pressure ~ 100 mm Hg. |
(2) Intravenous techniques. Con: more onerous use;
prolonged/unpredictable awakening (mitigated by
target-controlled infusion [TCI]; short-acting,
infusion duration-insensitive drugs [e.g.,
propofol, remifentanil]). Pro: intact CBF-CMRo2
coupling; ↓CBF/ICP/brain bulk; propofol blunts
N2O cerebrostimulation. Recommendation: use for
cases with high risk of ICP/brain bulk problems or
intraoperative cerebral ischemia; use TCI and
short-acting drugs.
 Management of increases in ICP and brain bulk
(Table-2)
Management of increases in ICP and brain bulk
(Table-2)
Other measures. When CNS and hemodynamic arousal are
evident despite adequate anesthesia/analgesia,
consider sympatholysis (esmolol, 0.5 to 1 mg/kg;
labetalol, 0.075 to 0.15 mg/kg; clonidine, 1 to 1.5
mcg/kg).
Antibioprophylaxis. Oxacillin or second-generation
cephalosporin before skin incision.
Fluid therapy. Goals: normovolemia, normotension,
normoglycemia, hematocrit approximately 30%, mild
hyperosmolality (<320 mOsm/L at end of procedure).
Recommendations: avoid glucose-containing solutions,
Ringer's lactate (hypo-osmolar); use 0.9% sodium
chloride (NaCl) or 6% hydroxyethyl starch.
 Emergence from anesthesia causes respiratory,
cardiovascular, metabolic/endocrine, and neurologic
changes. Emergence is associated with hemodynamic
arousal lasting 10 to 25 minutes, weakly correlating
with rises in oxygen consumption and mediated by
elevated catecholamine levels and nociceptive
stimuli. Treatment: antinociception, sympatholysis.
Oxygen consumption is increased (up to 5 times) by
rewarming (shivering/nonshivering thermogenesis) and
pain. As a result of all of these factors, 20% of
elective craniotomy patients develop raised ICP in
the early postoperative period. Systemic
hypertension is frequent and has been associated
with an increased risk of postoperative intracranial
hemorrhage.
Emergence from anesthesia causes respiratory,
cardiovascular, metabolic/endocrine, and neurologic
changes. Emergence is associated with hemodynamic
arousal lasting 10 to 25 minutes, weakly correlating
with rises in oxygen consumption and mediated by
elevated catecholamine levels and nociceptive
stimuli. Treatment: antinociception, sympatholysis.
Oxygen consumption is increased (up to 5 times) by
rewarming (shivering/nonshivering thermogenesis) and
pain. As a result of all of these factors, 20% of
elective craniotomy patients develop raised ICP in
the early postoperative period. Systemic
hypertension is frequent and has been associated
with an increased risk of postoperative intracranial
hemorrhage.
 Aims of emergence. Maintain intra- or extracranial
homeostasis (MAP-CPP-CBF-ICP, CMRo2, Paco2, Pao2,
temperature). Avoid factors leading to intracranial
bleeding (e.g., coughing, intratracheal suctioning,
ventilator fight, ↑ blood pressure). The patient
should be calm, cooperative, and responsive to
verbal commands soon after emergence.
Aims of emergence. Maintain intra- or extracranial
homeostasis (MAP-CPP-CBF-ICP, CMRo2, Paco2, Pao2,
temperature). Avoid factors leading to intracranial
bleeding (e.g., coughing, intratracheal suctioning,
ventilator fight, ↑ blood pressure). The patient
should be calm, cooperative, and responsive to
verbal commands soon after emergence.
| Table-6. Early vs. delayed awakening: pros and
cons |
|
Early Awakening |
Delayed Awakening |
| Pros |
Pros |
| Earlier neurologic examination and reintervention
|
Less risk of hypoxemia and/or hypercarbia |
| Baseline neurology for subsequent examinations |
Better respiratory, hemodynamic control |
| Less hypertension, catecholamine burst |
Easier to transfer to the ICU |
| Performed by anesthesiologist who knows patient
|
Stabilization in same state as during surgery |
| Surgery/recovery period separated,
↓costs |
↑Better late hemostasis |
| Cons |
Cons |
| Increased risk of hypoxemia, hypercarbia |
Less neurologic monitoring |
| Respiratory monitoring during transfer to ICU |
More hypertension, catecholamine release→↑bleeding |
| ICU, intensive care unit.
|
 Early versus late emergence.
Ideal: rapid emergence to permit early assessment of
surgical results and postoperative neurologic
follow-up. However, early emergence is still not
appropriate for some categories of patients.
Early versus late emergence.
Ideal: rapid emergence to permit early assessment of
surgical results and postoperative neurologic
follow-up. However, early emergence is still not
appropriate for some categories of patients.
 Indications for late emergence. Obtunded
consciousness or inadequate airway control
preoperatively; intraoperative catastrophe;
significant risk of brain edema,
↑ICP, or
deranged intracerebral hemo- or homeostasis
postoperatively. Risk factors for latter: long (>6
hours) and extensive surgery (particularly with
bleeding), repeat surgery, surgery involving or
close to vital brain areas, and
surgery associated with significant brain ischemia
(e.g., long vascular clipping times, extensive
retractor pressure). If delayed emergence is chosen,
adequate sedation and analgesia should be ensured,
preferably with short-acting drugs.
Indications for late emergence. Obtunded
consciousness or inadequate airway control
preoperatively; intraoperative catastrophe;
significant risk of brain edema,
↑ICP, or
deranged intracerebral hemo- or homeostasis
postoperatively. Risk factors for latter: long (>6
hours) and extensive surgery (particularly with
bleeding), repeat surgery, surgery involving or
close to vital brain areas, and
surgery associated with significant brain ischemia
(e.g., long vascular clipping times, extensive
retractor pressure). If delayed emergence is chosen,
adequate sedation and analgesia should be ensured,
preferably with short-acting drugs.
|
Table -7. Check-list before trying an early landing
|
| Adequate preoperative state of consciousness |
| Cardiovascular stability, normal body temperature,
and adequate oxygenation |
| Limited brain surgery, no major brain laceration |
| No extensive posterior fossa surgery involving
cranial nerves IX-XII |
| No major arteriovenous malformation removal
(avoiding malignant postoperative edema) |
 Preconditions for early emergence. Anesthesiologic:
should be planned (Table-7); use pharmacologically
adequate anesthetic technique for early awakening;
pay meticulous attention to intraoperative
homeostasis (oxygenation, temperature, intravascular
volume, cardiovascular function, CNS metabolism);
avoid trauma of mechanical brain retraction
(pharmacologic ICP/brain bulk control; see Table-5). Neurosurgical: minimization of blood loss
(obsessive hemostasis); minimal surgical
invasiveness (microsurgery, small operative fields).
Craniotomy may be painful after the operation.
Postoperative analgesia should be anticipated before
awakening, especially if remifentanil is used for
maintenance. Under these conditions, early emergence
can be associated with less hemodynamic, metabolic,
and endocrine activation than for delayed emergence.
Preconditions for early emergence. Anesthesiologic:
should be planned (Table-7); use pharmacologically
adequate anesthetic technique for early awakening;
pay meticulous attention to intraoperative
homeostasis (oxygenation, temperature, intravascular
volume, cardiovascular function, CNS metabolism);
avoid trauma of mechanical brain retraction
(pharmacologic ICP/brain bulk control; see Table-5). Neurosurgical: minimization of blood loss
(obsessive hemostasis); minimal surgical
invasiveness (microsurgery, small operative fields).
Craniotomy may be painful after the operation.
Postoperative analgesia should be anticipated before
awakening, especially if remifentanil is used for
maintenance. Under these conditions, early emergence
can be associated with less hemodynamic, metabolic,
and endocrine activation than for delayed emergence.
 Differential diagnosis of unplanned delayed
emergence. Within 10 to 20 minutes of cessation of
pharmacologically adequate anesthesia with
short-acting agents, the patient should be awake
enough to obey simple verbal commands. If not,
consider and treat or rule out nonanesthetic causes
(seizure, cerebral edema, intracranial hematoma,
pneumocephalus, vessel occlusion/ischemia, metabolic
or electrolyte disturbances). Suspected opioid
overhang (fentanyl or sufentanil): try carefully
titrated antagonization with small doses of naloxone
or naltrexone.
Differential diagnosis of unplanned delayed
emergence. Within 10 to 20 minutes of cessation of
pharmacologically adequate anesthesia with
short-acting agents, the patient should be awake
enough to obey simple verbal commands. If not,
consider and treat or rule out nonanesthetic causes
(seizure, cerebral edema, intracranial hematoma,
pneumocephalus, vessel occlusion/ischemia, metabolic
or electrolyte disturbances). Suspected opioid
overhang (fentanyl or sufentanil): try carefully
titrated antagonization with small doses of naloxone
or naltrexone.
 Neurologic evaluation. Perform a baseline simple
examination to assess motor responses of arms and
legs, size of pupils and reactivity to light,
adequate understanding of simple words and verbal
response, and orientation to time and space.
Neurologic evaluation. Perform a baseline simple
examination to assess motor responses of arms and
legs, size of pupils and reactivity to light,
adequate understanding of simple words and verbal
response, and orientation to time and space.
 Specific anesthetic management
Specific anesthetic management
 Predicted difficult airway. Avoiding hypoxia is more
important than preventing ICP increases. Method of
choice: fiberoptic intubation. Technique:
well-prepared, informed, cooperative patient; good
local anesthesia (nasopharynx, airways);
supplemental judicious light sedation (bolus
midazolam, 0.5 to 1 mg ± fentanyl, 25 to 50 mcg;
alternatively: low-dose propofol infusion at 1 to 2
mg/kg/hour) but avoid deep sedation and hypercapnia;
treat
hypertension promptly (esmolol, labetalol,
clonidine).
Predicted difficult airway. Avoiding hypoxia is more
important than preventing ICP increases. Method of
choice: fiberoptic intubation. Technique:
well-prepared, informed, cooperative patient; good
local anesthesia (nasopharynx, airways);
supplemental judicious light sedation (bolus
midazolam, 0.5 to 1 mg ± fentanyl, 25 to 50 mcg;
alternatively: low-dose propofol infusion at 1 to 2
mg/kg/hour) but avoid deep sedation and hypercapnia;
treat
hypertension promptly (esmolol, labetalol,
clonidine).
 Infectious tumors (abscesses) are part of the
differential diagnosis of supratentorial mass
lesions. They are often accompanied by low-grade
fever. Risk factors: contiguous infections (sinus,
ear); right-to-left cardiac shunt; immunosuppression
(extrinsic/intrinsic); intravenous drug abuse.
Initial treatment: antibiotics (infection);
corticosteroids (brain swelling). Definitive
diagnosis/treatment: craniotomy, abscess aspiration.
Surgical and anesthetic management: as for
supratentorial neoplasms; aseptic precautions and
sterile technique are vital for immunocompromised
patients with acquired immunodeficiency syndrome.
Note the association between human immunodeficiency
virus infection and cerebral non-Hodgkin's
lymphomas.
Infectious tumors (abscesses) are part of the
differential diagnosis of supratentorial mass
lesions. They are often accompanied by low-grade
fever. Risk factors: contiguous infections (sinus,
ear); right-to-left cardiac shunt; immunosuppression
(extrinsic/intrinsic); intravenous drug abuse.
Initial treatment: antibiotics (infection);
corticosteroids (brain swelling). Definitive
diagnosis/treatment: craniotomy, abscess aspiration.
Surgical and anesthetic management: as for
supratentorial neoplasms; aseptic precautions and
sterile technique are vital for immunocompromised
patients with acquired immunodeficiency syndrome.
Note the association between human immunodeficiency
virus infection and cerebral non-Hodgkin's
lymphomas.
 Craniofacial/skull base surgery. Increasingly used
for orbital, posterior nasal sinus wall tumors.
Particularities: complex, multidisciplinary surgery; tracheostomy/oral intubation frequent. Extensive
bony involvement→↑ bleeding, hemorrhagic
diathesis, venous air embolism (head-up position).
Sensory ± motor neurophysiologic cranial nerve
monitoring is common (motor monitoring: avoid
neuromuscular blockade). Repeat procedures may be
necessary and a difficult intubation (skull base
exposure requires extensive temporalis muscle
mobilization, which can lead to mandibular
pseudoankylosis and limited mouth opening) can
result.
Craniofacial/skull base surgery. Increasingly used
for orbital, posterior nasal sinus wall tumors.
Particularities: complex, multidisciplinary surgery; tracheostomy/oral intubation frequent. Extensive
bony involvement→↑ bleeding, hemorrhagic
diathesis, venous air embolism (head-up position).
Sensory ± motor neurophysiologic cranial nerve
monitoring is common (motor monitoring: avoid
neuromuscular blockade). Repeat procedures may be
necessary and a difficult intubation (skull base
exposure requires extensive temporalis muscle
mobilization, which can lead to mandibular
pseudoankylosis and limited mouth opening) can
result.
II. Anesthesia for intracranial hematomas
 General considerations. The effects of intracranial
hematomas on neurostatus and ICP depend particularly
on the speed with which they arise. Slow: chronic
subdural hematomas- subtle neurologic signs, small
↑ICP; anesthetic technique: similar to
supratentorial tumors. Most often seen in elderly
patients (>70 years). Fast: acute epidural (e.g.,
traumatic), subdural, or intracerebral hematoma-massive neurologic impairment, potentially acutely
life-threatening ↑ICP; anesthetic technique:
aggressive reduction of ICP and measures to preserve brain
oxygenation and perfusion, followed by urgent
surgical decompression. Situation frequently seen in
head trauma or due to anticoagulation or antiplatelet agents. Coagulation should be corrected
before surgery (factors II, VII, IX, and X, and
vitamin K for patients treated with vitamin K
antagonists; platelet transfusion for patients
taking clopidogrel.
General considerations. The effects of intracranial
hematomas on neurostatus and ICP depend particularly
on the speed with which they arise. Slow: chronic
subdural hematomas- subtle neurologic signs, small
↑ICP; anesthetic technique: similar to
supratentorial tumors. Most often seen in elderly
patients (>70 years). Fast: acute epidural (e.g.,
traumatic), subdural, or intracerebral hematoma-massive neurologic impairment, potentially acutely
life-threatening ↑ICP; anesthetic technique:
aggressive reduction of ICP and measures to preserve brain
oxygenation and perfusion, followed by urgent
surgical decompression. Situation frequently seen in
head trauma or due to anticoagulation or antiplatelet agents. Coagulation should be corrected
before surgery (factors II, VII, IX, and X, and
vitamin K for patients treated with vitamin K
antagonists; platelet transfusion for patients
taking clopidogrel.
 Anesthetic management of acute intracranial hematoma
Anesthetic management of acute intracranial hematoma
 Induction.
Induction.
a. Basics. Ensure oxygenation and then secure airway
and hyperventilate with 100% oxygen.
Swift, atraumatic intubation (always dangerous if a
fractured cervical spine is suspected or confirmed
by x-ray); aim for a minimal ICP rise by avoiding
coughing and arterial hypertension due to light
anesthesia. In polytraumatized, hypotensive, and
hypovolemic patients, one should decrease hypnotic,
analgesic doses and restore circulating volume. If
the patient has a full stomach, use aspiration
prophylaxis and cricoid pressure (cautiously if
suspected fractured cervical spine).
b. Pharmacologic range of options. Intubation without
further use of drugs in the deeply unconscious
patient; judicious sedative use (e.g., etomidate,
0.2 to 0.5 mg/kg; propofol, 0.5 to 1.5 mg/kg; or
thiopental, 2 to 4 mg/kg) with myorelaxation for a
semiconscious, struggling patient; classical rapid sequence induction for the (still) conscious
and stable patient. Controversy: what myorelaxant
scheme to use? Succinylcholine, perhaps preceded by
a small dose of nondepolarizing myorelaxant, remains
the classical and time-tested scheme.
c. Control of ICP and brain swelling. Next priority
after securing ventilation and airway; should be
started as early as possible and continued through
to intensive care treatment. Start with large doses
of mannitol, 0.7 to 1.4 g/kg (Table -2).
 Anesthesia maintenance. Aims: control of ICP and
brain swelling; maintenance of cerebral perfusion
and oxygenation by matching CMRo2 and CBF.
Anesthesia maintenance. Aims: control of ICP and
brain swelling; maintenance of cerebral perfusion
and oxygenation by matching CMRo2 and CBF.
a. Monitoring
(1) Cardiovascular monitoring for these frequently
hemodynamically unstable patients should include
invasive arterial pressure monitoring, preferably
commenced before induction (close hemodynamic
control, repeated laboratory determinations).
Electrocardiographic monitoring: interactions
between brain damage and myocardial injury, risk of
arrhythmias.
(2) ICP monitoring. Generally installed once
hematoma is evacuated, mainly for use in intensive
care unit.
(3) Laboratory analyses. Blood gas analysis (acid-base
balance, ventilation, etc.); glucose (hyperglycemia
and brain ischemia); coagulation profile (brain
tissue damage→↑ circulating thromboplastin);
blood osmolality as guidance for use of osmotic
diuretics (e.g., with mannitol, maximum should be
320 mOsm/kg).
 Anesthetic technique. Intravenous anesthetics (→↓CMRo2,
↓CBF,↑CVR) are the mainstay of
anesthesia for acute intracranial hematoma. Volatile
anesthetics are not recommended because of risk of
→↑ICP/brain tension (to the point of acute
transtentorial/craniotomy herniation, even with
preexisting hypocapnia) and much smaller CMRo2
reduction and neuroprotection against focal ischemia
than with intravenous anesthetics (propofol,
barbiturates). The following different situations
must be evaluated and treated:
Anesthetic technique. Intravenous anesthetics (→↓CMRo2,
↓CBF,↑CVR) are the mainstay of
anesthesia for acute intracranial hematoma. Volatile
anesthetics are not recommended because of risk of
→↑ICP/brain tension (to the point of acute
transtentorial/craniotomy herniation, even with
preexisting hypocapnia) and much smaller CMRo2
reduction and neuroprotection against focal ischemia
than with intravenous anesthetics (propofol,
barbiturates). The following different situations
must be evaluated and treated:
(1) Deep coma and signs of brain herniation:
myorelaxation and repeated small doses of thiopental
titrated to blood pressure
(2) Coma but no sign of herniation, increased ICP:
propofol TCI or small doses of thiopental and
opioids titrated to blood pressure; myorelaxation
(3) Conscious patient but mass effect on CT-scan:
rapid sequence induction, followed by propofol TCI,
opioids, and myorelaxation
 Cardiovascular control. Avoid arterial hypotension
(by using doses of intravenous anesthetics that are
too large) to prevent ↓CPP (↑cerebral ischemia
and/or reflex cerebral vasodilatation
→↑ICP
not controlled by hypocapnia [vasodilatory
cascade]). Controversy: control of arterial
hypertension and acute intracranial hematoma (Table-2): carefully balance maintenance of CPP to areas
of brain rendered ischemic due to compression by
hematoma against risk of more vasogenic brain edema
or bleeding. Jugular venous bulb oxygen saturation
monitoring may help assess adequacy of global CPP.
btPo2 can help assess adequacy of local O2 delivery.
Globally adequate CPP does not rule out regional CPP
inadequacies ↑regional ischemia. If arterial
pressure requires reduction, first improve analgesia
(i.e., opioids) and/or depth of anesthesia
(propofol, barbiturates, etomidate) before
instituting specific antihypertensive treatment
(usually antisympathetic drugs [e.g., esmolol,
labetalol, clonidine]). Avoid cerebral vasodilators.
Decrease blood pressure no >15% to 20%. Anticipate
severe hypotension after brain decompression due to
disappearance of the Cushing response: rapid fluid
loading, neosynephrine, noradrenaline, or
epinephrine ready to use.
Cardiovascular control. Avoid arterial hypotension
(by using doses of intravenous anesthetics that are
too large) to prevent ↓CPP (↑cerebral ischemia
and/or reflex cerebral vasodilatation
→↑ICP
not controlled by hypocapnia [vasodilatory
cascade]). Controversy: control of arterial
hypertension and acute intracranial hematoma (Table-2): carefully balance maintenance of CPP to areas
of brain rendered ischemic due to compression by
hematoma against risk of more vasogenic brain edema
or bleeding. Jugular venous bulb oxygen saturation
monitoring may help assess adequacy of global CPP.
btPo2 can help assess adequacy of local O2 delivery.
Globally adequate CPP does not rule out regional CPP
inadequacies ↑regional ischemia. If arterial
pressure requires reduction, first improve analgesia
(i.e., opioids) and/or depth of anesthesia
(propofol, barbiturates, etomidate) before
instituting specific antihypertensive treatment
(usually antisympathetic drugs [e.g., esmolol,
labetalol, clonidine]). Avoid cerebral vasodilators.
Decrease blood pressure no >15% to 20%. Anticipate
severe hypotension after brain decompression due to
disappearance of the Cushing response: rapid fluid
loading, neosynephrine, noradrenaline, or
epinephrine ready to use.
 Emergence. Patients with acute cerebral hematoma
have significant brain injury with
significant actual and potential brain swelling.
They should therefore undergo slow weaning and
delayed extubation in the neurointensive care unit.
Chronic subdural hematoma patients frequently have
minimal neurologic impairment preoperatively and can
therefore often be awakened and their tracheas
extubated immediately after surgery.
Emergence. Patients with acute cerebral hematoma
have significant brain injury with
significant actual and potential brain swelling.
They should therefore undergo slow weaning and
delayed extubation in the neurointensive care unit.
Chronic subdural hematoma patients frequently have
minimal neurologic impairment preoperatively and can
therefore often be awakened and their tracheas
extubated immediately after surgery.
III. Conclusions
The main objectives of anesthesia for excision of a
cerebral tumor include the following:
 Preserving uninjured cerebral territories by global
maintenance of cerebral homeostasis and
cardiovascular stability as well as neuroprotection.
Preserving uninjured cerebral territories by global
maintenance of cerebral homeostasis and
cardiovascular stability as well as neuroprotection.
 Balancing CBF autoregulation and MAP and preserving
cerebral vasoreactivity to Paco2.
Balancing CBF autoregulation and MAP and preserving
cerebral vasoreactivity to Paco2.
 Achieving and maintaining brain relaxation by means
of:
Achieving and maintaining brain relaxation by means
of:
↓CMRo2, CBF, and CBV
 moderate hyperventilation (Paco2 ~ 35 mm Hg)
moderate hyperventilation (Paco2 ~ 35 mm Hg)
 strict maintenance of CPP
strict maintenance of CPP
 osmotherapy
osmotherapy
 CSF drainage
CSF drainage
Timely awakening to facilitate early and continuing
neurologic assessment and permit prompt diagnosis
and treatment of complications.
|